Fishery Bulletin/U S Dept of Commerce National
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
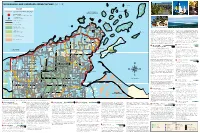
WLSSB Map and Guide
WISCONSIN LAKE SUPERIOR SCENIC BYWAY (WLSSB) DEVILS ISLAND NORTH TWIN ISLAND MAP KEY ROCKY ISLAND SOUTH TWIN ISLAND CAT ISLAND WISCONSIN LAKE SUPERIOR SCENIC BYWAY APOSTLE ISLANDS BEAR ISLAND NATIONAL LAKESHORE KIOSK LOCATION IRONWOOD ISLAND SCENIC BYWAY NEAR HERBSTER SAILING ON LAKE SUPERIOR LOST CREEK FALLS KIOSKS CONTAIN DETAILED INFORMATION ABOUT EACH LOCATION SAND ISLAND VISITOR INFORMATION OUTER ISLAND YORK ISLAND SEE REVERSE FOR COMPLETE LIST µ OTTER ISLAND FEDERAL HIGHWAY MANITOU ISLAND RASPBERRY ISLAND STATE HIGHWAY COUNTY HIGHWAY 7 EAGLE ISLAND NATIONAL PARKS ICE CAVES AT MEYERS BEACH BAYFIELD PENINSULA AND THE APOSTLE ISLANDS FROM MT. ASHWABAY & NATIONAL FOREST LANDS well as a Heritage Museum and a Maritime Museum. Pick up Just across the street is the downtown area with a kayak STATE PARKS K OAK ISLAND STOCKTON ISLAND some fresh or smoked fish from a commercial fishery for a outfitter, restaurants, more lodging and a historic general & STATE FOREST LANDS 6 GULL ISLAND taste of Lake Superior or enjoy local flavors at one of the area store that has a little bit of everything - just like in the “old (!13! RED CLIFF restaurants. If you’re brave, try the whitefish livers – they’re a days,” but with a modern flair. Just off the Byway you can MEYERS BEACH COUNTY PARKS INDIAN RESERVATION local specialty! visit two popular waterfalls: Siskiwit Falls and Lost Creek & COUNTY FOREST LANDS Falls. West of Cornucopia you will find the Lost Creek Bog HERMIT ISLAND Walk the Brownstone Trail along an old railroad grade or CORNUCOPIA State Natural Area. Lost Creek Bog forms an estuary at the take the Gil Larson Nature Trail (part of the Big Ravine Trail MICHIGAN ISLAND mouths of three small creeks (Lost Creek 1, 2, and 3) where System) which starts by a historic apple shed, continues RESERVATION LANDS they empty into Lake Superior at Siskiwit Bay. -

Apostle Islands National Lakehore Geologic Resources Inventory
Geologic Resources Inventory Scoping Summary Apostle Islands National Lakeshore Geologic Resources Division Prepared by Trista L. Thornberry-Ehrlich National Park Service August 7, 2010 US Department of the Interior The Geologic Resources Inventory (GRI) provides each of 270 identified natural area National Park System units with a geologic scoping meeting and summary (this document), a digital geologic map, and a geologic resources inventory report. The purpose of scoping is to identify geologic mapping coverage and needs, distinctive geologic processes and features, resource management issues, and monitoring and research needs. Geologic scoping meetings generate an evaluation of the adequacy of existing geologic maps for resource management, provide an opportunity to discuss park-specific geologic management issues, and if possible include a site visit with local experts. The National Park Service held a GRI scoping meeting for Apostle Islands National Lakeshore on July 20-21, 2010 both out in the field on a boating site visit from Bayfield, Wisconsin, and at the headquarters building for the Great Lakes Network in Ashland, Wisconsin. Jim Chappell (Colorado State University [CSU]) facilitated the discussion of map coverage and Bruce Heise (NPS-GRD) led the discussion regarding geologic processes and features at the park. Dick Ojakangas from the University of Minnesota at Duluth and Laurel Woodruff from the U.S. Geological Survey presented brief geologic overviews of the park and surrounding area. Participants at the meeting included NPS staff from the park and Geologic Resources Division; geologists from the University of Minnesota at Duluth, Wisconsin Geological and Natural History Survey, and U.S. Geological Survey; and cooperators from Colorado State University (see table 2). -

Apostle Islands National Seashore
Apostle Islands National Seashore David Speer & Phillip Larson October 2nd Fieldtrip Report Table of Contents Introduction 1 Stop 1: Apostle Island Boat Cruise 1 Stop 2: Coastal Geomorphology 5 Stop 3: Apostle Islands National Seashore Headquarters 12 Stop 4: Northern Great Lakes Visitors Center 13 Stop 5: Montreal River 16 Stop 6: Conclusion 17 Bibliography 18 Introduction: On October 2nd, 2006, I and the other Geomorphology students took a field excursion to the Apostle Islands and the south shore of Lake Superior. On this trip we made five stops along our journey where we noted various geomorphic features and landscapes of the Lake Superior region. We reached the south coast of Lake Superior in Bayfield, Wisconsin at around 10:00am. Here we made our first stop which included the entire boat trip we took which navigated through the various islands in the Apostle Island National Lakeshore. Our second stop was a beach on the shore of Lake Superior where we noted active anthropogenic and environmental changes to a shoreline. Stop three was at the visitor center for the Apostle Island National Lakeshore where we heard a short talk by the resident park ranger as he explained some of the unique features of the islands and why they should be protected. After that we proceeded to our fourth stop which was at the Northern Great Lakes Visitor Center. Here we learned, through various displays and videos, how the region had been impacted by various events over time. Finally our fifth stop was at the point where the Montreal River emptied into Lake Superior (Figure 1). -

22 AUG 2021 Index Acadia Rock 14967
19 SEP 2021 Index 543 Au Sable Point 14863 �� � � � � 324, 331 Belle Isle 14976 � � � � � � � � � 493 Au Sable Point 14962, 14963 �� � � � 468 Belle Isle, MI 14853, 14848 � � � � � 290 Index Au Sable River 14863 � � � � � � � 331 Belle River 14850� � � � � � � � � 301 Automated Mutual Assistance Vessel Res- Belle River 14852, 14853� � � � � � 308 cue System (AMVER)� � � � � 13 Bellevue Island 14882 �� � � � � � � 346 Automatic Identification System (AIS) Aids Bellow Island 14913 � � � � � � � 363 A to Navigation � � � � � � � � 12 Belmont Harbor 14926, 14928 � � � 407 Au Train Bay 14963 � � � � � � � � 469 Benson Landing 14784 � � � � � � 500 Acadia Rock 14967, 14968 � � � � � 491 Au Train Island 14963 � � � � � � � 469 Benton Harbor, MI 14930 � � � � � 381 Adams Point 14864, 14880 �� � � � � 336 Au Train Point 14969 � � � � � � � 469 Bete Grise Bay 14964 � � � � � � � 475 Agate Bay 14966 �� � � � � � � � � 488 Avon Point 14826� � � � � � � � � 259 Betsie Lake 14907 � � � � � � � � 368 Agate Harbor 14964� � � � � � � � 476 Betsie River 14907 � � � � � � � � 368 Agriculture, Department of� � � � 24, 536 B Biddle Point 14881 �� � � � � � � � 344 Ahnapee River 14910 � � � � � � � 423 Biddle Point 14911 �� � � � � � � � 444 Aids to navigation � � � � � � � � � 10 Big Bay 14932 �� � � � � � � � � � 379 Baby Point 14852� � � � � � � � � 306 Air Almanac � � � � � � � � � � � 533 Big Bay 14963, 14964 �� � � � � � � 471 Bad River 14863, 14867 � � � � � � 327 Alabaster, MI 14863 � � � � � � � � 330 Big Bay 14967 �� � � � � � � � � � 490 Baileys -

Apostle Islands National Lakeshore Geologic Resources Inventory Report
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science Apostle Islands National Lakeshore Geologic Resources Inventory Report Natural Resource Report NPS/NRSS/GRD/NRR—2015/972 ON THIS PAGE An opening in an ice-fringed sea cave reveals ice flows on Lake Superior. Photograph by Neil Howk (National Park Service) taken in winter 2008. ON THE COVER Wind and associated wave activity created a window in Devils Island Sandstone at Devils Island. Photograph by Trista L. Thornberry-Ehrlich (Colorado State University) taken in summer 2010. Apostle Islands National Lakeshore Geologic Resources Inventory Report Natural Resource Report NPS/NRSS/GRD/NRR—2015/972 Trista L. Thornberry-Ehrlich Colorado State University Research Associate National Park Service Geologic Resources Division Geologic Resources Inventory PO Box 25287 Denver, CO 80225 May 2015 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate comprehensive information and analysis about natural resources and related topics concerning lands managed by the National Park Service. The series supports the advancement of science, informed decision-making, and the achievement of the National Park Service mission. The series also provides a forum for presenting more lengthy results that may not be accepted by publications with page limitations. -

=- ::.: III ~ III ..II L ~ • III ~
z o VI - - '" 0 III > ~ III :E: -VI ..~ VI • III =- ::.: III ~ III ..II l ~ • III ~ - - ---- ------------- GREAT LAKES FISHERY COMMISSION GREAT LAKES FISHERY COMMISSION MEMBERS - 1911 Established by Convention between Canada and the United States for the Conservation of Great Lakes Fishery Resources CANADA UNITED STATES E. W. Burridge W. M. Lawrence F. E. J. Fry N. P. Reed C. J. Kerswill Claude Ver Duin ANNUAL REPORT K. H. Loftus L. P. Voigt for the year 1977 SECRETARIAT C. M. Fetterolf, Jr., Executive Secretary 1451 Green Road A. K. Lamsa, Assistant Executive Secretary Ann Arbor, Michigan, J. Herbert, Fishery Biologist U. S. A. T. C. Woods, Secretary 1980 CONTENTS LETTER OF TRANSMITTAL In accordance with ArtiCle IX of the Convention on INTRODUCTION . 1 Great Lakes Fisheries, I take pleasure in submitting ANNUAL MEETING PROCEEDINGS. 2 to the Contracting Parties an Annual Report of the INTERIM MEETING PROCEEDINGS. 9 activities of the Great Lakes Fishery Commission in 1977. APPENDICES A. Summary of Management and Research . 16 Respectfully, B. Summary of Trout, Splake, and Salmon Plantings 25 C. Sea Lamprey Control in the United States . 63 D. Sea Lamprey Control in Canada. 90 L. P. Voigt, Chairman E. Alternative Methods of Sea Lamprey Control. 96 F. Registration-oriented Research on Lampricides, 1977 103 G. Administrative Report for 1977. 110 ~ ANNUAL REPORT FOR 1977 INTRODUCTION A Convention on Great Lakes Fisheries, ratified by the Governments of the United States and Canada in 1955 provided for the establishment of the Great Lakes Fishery Commission. The Commission was given the responsibilities of formulating and coordinating fishery research and management programs, advising govern ments on measures to improve the fisheries, and implementing a program to control the sea lamprey. -

A Data Compilation and Assessment of Coastal Wetlands of Wisconsin's Great Lakes
A Data Compilation and Assessment of Coastal Wetlands of Wisconsin's Great Lakes March 2000 Primary Author: Nicole Merryfield Contributing Authors: Eric Epstein, Andrew Galvin, Emmet Judziewicz, William Smith Natural Heritage Inventory Program Bureau of Endangered Resources Wisconsin Department of Natural Resources P.O. Box 7921 Madison, WI 53707 Publication Number: PUBL-ER-002 00 Please write to the above address for copies of this report. Cover photos by Eric Epstein, Emmet Judziewicz, and Thomas Meyer, Bureau of Endangered Resources. Scanning completed by Robert Queen. 2 A Data Compilation and Assessment ACKNOWLEDGMENTS Funding for this project was provided by the Department of Administration's Coastal Management Program and the Department of Natural Resources Endangered Resources Fund. We gratefully acknowledge this support. Many individuals provided valuable insight, advice, and technical information necessary to complete this study. We wish to extend our thanks to the following persons for their assistance: i Department of Natural Resources Staff: Julie Bleser, Eric Epstein, Andy Galvin, Bob Hay, Randy Hoffman, Kelly Kearns, Ann Lacy, Calvin Lawrence, Betty Les, Emmet Judziewicz, Mark Martin, Sumner Matteson, Thomas Meyer, Mitch Moline, Dave Sample, Sonia Slemrod, William Smith, Lois Stoerzer, Kristin Westad, Darrell Zastrow, Barb Zellmer i Conservation Organizations: Roy Aiken from Door County Property Owners, Mike Grimm of The Nature Conservancy i Government Agencies: Dea Larsen Converse and Diana Toledo of the Wisconsin Coastal Management Program of Department of Administration, Candice Kasprzak and Mark Walter of the Bay-Lake Regional Planning Commission, Larry Leitner and John McDugle of the Southeastern Wisconsin Regional Planning Commission, Joel Trick of the U.S. -

2016 Around the Archipelago
National Park Service Apostle Islands National Lakeshore U.S. Department of the Interior The official newspaper - 2016 Around the Archipelago Centennial Events The National Park Service Turns 100 at Apostle Islands IT WAS KIND OF A ROCKY START. IN THE 44 YEARS National Lakeshore following the establishment of Yellowstone as the world’s first “national park” in 1872, the United States authorized 34 additional Apostle Islands National Lakeshore has national parks and monuments, but no single agency provided planned a number of special events to unified management of the varied federal park lands. celebrate the NPS Centennial including: On August 25, 1916, President Woodrow Wilson signed the act Chequamegon Bay Birding and Nature creating the National Park Service (NPS), a new federal bureau in Festival. May 19-21. The 10th annual the Department of the Interior responsible for protecting the 35 festival will include numerous guided trips national parks and monuments then managed by the department in the park and a keynote presentation on and those yet to be established. This “Organic Act” states that the NPS Centennial by assistant chief of “the Service thus established shall promote and regulate the use interpretation Neil Howk. of the Federal areas known as national parks, monuments and Mural Dedication in Ashland. June reservations…by such means and measures as…to conserve the 25th at 11 am. A permanent, outdoor, scenery and the natural and historic objects and the wild life therein public mural celebrating the Apostle Islands and to provide for the enjoyment of the same in such manner and National Lakeshore will be completed and by such means as will leave them unimpaired for the enjoyment of dedicated in a public ceremony in the park’s future generations.” newest gateway community of Ashland, WI. -

Stockton Island
National Park Service Apostle Islands U.S. Department of the Interior Apostle Islands National Lakeshore Wisconsin Stockton Island Tracks in the Sand What would the Apostle Islands be without sand? They sit on a foundation of billion year old sandstone. Sand is a major component of the “till” left behind when the glaciers melted 10,000 years ago. Today, wind and waves sculpt exposed shorelines and carry sand grains to protected shores to form beaches. No island is blessed with more spectacular and diverse sandy areas than Stockton. The 10,054 acre island features sand beaches at Quarry Bay, Presque Isle Bay, and Julian Bay. The bridge of sand connecting Presque Isle Point to the rest of Stockton Island is an outstanding example of a geologic feature called a “tombolo”. The tombolo is home to a variety of plant communities (bogs, dunes, lagoons, savannahs, and pine forests), the park’s largest campgound, and the “singing sands” of Julian Bay. People feel called to beaches everywhere, but at Julian Bay, where the “singing” sand squeaks underfoot, the call can actually be heard. Bearprints Bear tracks on the beach have become a common and Quarry Bay. “Skar’s” campground visits in sight on Stockton Island. The island supports one of search of “easy pickings” became increasingly the most concentrated populations of black bears in frequent and menacing in August as the blueberry the world. A study of Stockton Island bears revealed crop waned. After using every available nonlethal that the population grew from two animals in 1984 to tool to discourage Skar’s aggressive behavior, park a high of 31 non-cub bears in 1994. -

Rewilding the Islands: Nature, History, and Wilderness at Apostle
Rewilding the Islands: Nature, History, and Wilderness at Apostle Islands National Lakeshore by James W. Feldman A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (History) at the UNIVERSITY OF WISCONSIN-MADISON 2004 © Copyright by James W. Feldman 2004 All Rights Reserved i For Chris ii TABLE OF CONTENTS ACKNOWLEDGEMENTS iii INTRODUCTION Wilderness and History 1 CHAPTER ONE Dalrymple’s Dream: The Process of Progress in the Chequamegon Bay 20 CHAPTER TWO Where Hemlock came before Pine: The Local Geography of the Chequamegon Bay Logging Industry 83 CHAPTER THREE Stability and Change in the Island Fishing Industry 155 CHAPTER FOUR Consuming the Islands: Tourism and the Economy of the Chequamegon Bay, 1855-1934 213 CHAPTER FIVE Creating the Sand Island Wilderness, 1880-1945 262 CHAPTER SIX A Tale of Two Parks: Rewilding the Islands, 1929-1970 310 CHAPTER SEVEN Conclusion: The Rewilding Dilemma 371 SELECTED BIBLIOGRAPHY 412 iii ACKNOWLEDGEMENTS *** One of the chief advantages of studying environmental history is that you can go hiking, camping, and exploring in beautiful places and call it research. By choosing the history of the Apostle Islands for a dissertation topic, I even added kayaking and sailing to my list of research activities. There were many more hours spent in libraries, but those weren’t quite as memorable. All graduate students should have topics like this one. As I cast about for a dissertation topic, I knew that I wanted a place-based subject that met two requirements: It needed to be about a place to which I had a personal connection, and it needed to apply to a current, ongoing policy question or environmental issue. -

Stockton Island
National Park Service Apostle Islands U.S. Department of the Interior Apostle Islands National Lakeshore Wisconsin Stockton Island Tracks in the Sand “Strolling the singing sand beach with views of the Northwoods Caribbean can’t be beat!” What would the Apostle Islands be without sand? No island is blessed with more spectacular and diverse sandy areas than Stockton. The 10,054 acre island features sand beaches at Quarry Bay, Presque Isle Bay, and Julian Bay. The bridge of sand connecting Presque Isle Point to the rest of Stockton Island is an outstanding example of a geologic feature called a “tombolo”. The tombolo is home to a variety of plant communities (bogs, dunes, lagoons, savannahs, and pine forests), the park’s largest campgound, and the “singing sands” of Julian Bay. People everywhere feel the call of the beach, but at Julian Bay, the call of the “singing” sand can actually be heard. Bear Prints “I dreamt of a bear while sleeping in my tent. In the end roamed between the camping areas at Presque morning there was a bear print next to my tent.” Isle and Quarry Bay. “Skar’s” campground visits in search of “easy pickings” slowly became more Stockton Island supports a resident population frequent and menacing. After using every available of black bears. A study of Stockton Island bears nonlethal tool to discourage Skar’s aggressive revealed that the population grew from two animals behavior, park rangers were forced to shoot him. in 1984 to a high of 31 non-cub bears in 1994. DNA Visitors must take precautions to help keep this analysis of bear hair samples collected in 2002 from happening again. -

Apostle Islands NL Wilderness Newspaper Article
National Park Service Park News U.S. Department of the Interior The official newspaper of Around the Archipelago Apostle Islands National Lakeshore Spring, Summer, Fall 2005 New Apostle Islands Wilderness Honors - Gaylord Nelson Wilderness ...there is not another collection of islands of this Management significance within the continental boundaries of the Now that the Gaylord Nelson Wilderness United States. I think it is tremendously important Area has been officially established, what that this collection of islands be preserved.” impacts will that have on how the National - Gaylord Nelson Park Service (NPS) manages the area? Since it is NPS policy to assure that With the stroke of a pen, on December 8, 2004, President management actions do not diminish the George W. Bush approved legislation designating 80% of the land wilderness suitability of an area possessing area of Wisconsin’s Apostle Islands National Lakeshore as wilderness characteristics pending federally protected wilderness. The new wilderness area – Congressional action, most of the Apostle Wisconsin’s largest by far – honors former Governor and U.S. Islands National Lakeshore has been Senator, Gaylord Nelson. This new addition to the National managed essentially as wilderness since Wilderness Preservation System will be known as the Gaylord 1989. This means that changes will be Nelson Wilderness. The designation guarantees that the present nearly imperceptible. One tangible change management style of Apostle Islands National Lakeshore will be will be the removal of picnic tables from 13 maintained in the future - emphasizing continued motorized boat campsites located in the new wilderness access to the mostly-wild islands, but no motorized travel on the area, since NPS policy precludes picnic tables in wilderness.