Regulation of the Centriole Life Cycle by Mdm1 a Dissertation Submitted to the Department of Biology and the Committee on Gradua
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Supplemental Information Proximity Interactions Among Centrosome
Current Biology, Volume 24 Supplemental Information Proximity Interactions among Centrosome Components Identify Regulators of Centriole Duplication Elif Nur Firat-Karalar, Navin Rauniyar, John R. Yates III, and Tim Stearns Figure S1 A Myc Streptavidin -tubulin Merge Myc Streptavidin -tubulin Merge BirA*-PLK4 BirA*-CEP63 BirA*- CEP192 BirA*- CEP152 - BirA*-CCDC67 BirA* CEP152 CPAP BirA*- B C Streptavidin PCM1 Merge Myc-BirA* -CEP63 PCM1 -tubulin Merge BirA*- CEP63 DMSO - BirA* CEP63 nocodazole BirA*- CCDC67 Figure S2 A GFP – + – + GFP-CEP152 + – + – Myc-CDK5RAP2 + + + + (225 kDa) Myc-CDK5RAP2 (216 kDa) GFP-CEP152 (27 kDa) GFP Input (5%) IP: GFP B GFP-CEP152 truncation proteins Inputs (5%) IP: GFP kDa 1-7481-10441-1290218-1654749-16541045-16541-7481-10441-1290218-1654749-16541045-1654 250- Myc-CDK5RAP2 150- 150- 100- 75- GFP-CEP152 Figure S3 A B CEP63 – – + – – + GFP CCDC14 KIAA0753 Centrosome + – – + – – GFP-CCDC14 CEP152 binding binding binding targeting – + – – + – GFP-KIAA0753 GFP-KIAA0753 (140 kDa) 1-496 N M C 150- 100- GFP-CCDC14 (115 kDa) 1-424 N M – 136-496 M C – 50- CEP63 (63 kDa) 1-135 N – 37- GFP (27 kDa) 136-424 M – kDa 425-496 C – – Inputs (2%) IP: GFP C GFP-CEP63 truncation proteins D GFP-CEP63 truncation proteins Inputs (5%) IP: GFP Inputs (5%) IP: GFP kDa kDa 1-135136-424425-4961-424136-496FL Ctl 1-135136-424425-4961-424136-496FL Ctl 1-135136-424425-4961-424136-496FL Ctl 1-135136-424425-4961-424136-496FL Ctl Myc- 150- Myc- 100- CCDC14 KIAA0753 100- 100- 75- 75- GFP- GFP- 50- CEP63 50- CEP63 37- 37- Figure S4 A siCtl -

Methods in and Applications of the Sequencing of Short Non-Coding Rnas" (2013)
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2013 Methods in and Applications of the Sequencing of Short Non- Coding RNAs Paul Ryvkin University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Bioinformatics Commons, Genetics Commons, and the Molecular Biology Commons Recommended Citation Ryvkin, Paul, "Methods in and Applications of the Sequencing of Short Non-Coding RNAs" (2013). Publicly Accessible Penn Dissertations. 922. https://repository.upenn.edu/edissertations/922 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/922 For more information, please contact [email protected]. Methods in and Applications of the Sequencing of Short Non-Coding RNAs Abstract Short non-coding RNAs are important for all domains of life. With the advent of modern molecular biology their applicability to medicine has become apparent in settings ranging from diagonistic biomarkers to therapeutics and fields angingr from oncology to neurology. In addition, a critical, recent technological development is high-throughput sequencing of nucleic acids. The convergence of modern biotechnology with developments in RNA biology presents opportunities in both basic research and medical settings. Here I present two novel methods for leveraging high-throughput sequencing in the study of short non- coding RNAs, as well as a study in which they are applied to Alzheimer's Disease (AD). The computational methods presented here include High-throughput Annotation of Modified Ribonucleotides (HAMR), which enables researchers to detect post-transcriptional covalent modifications ot RNAs in a high-throughput manner. In addition, I describe Classification of RNAs by Analysis of Length (CoRAL), a computational method that allows researchers to characterize the pathways responsible for short non-coding RNA biogenesis. -

Supplemental Information
Supplemental information Dissection of the genomic structure of the miR-183/96/182 gene. Previously, we showed that the miR-183/96/182 cluster is an intergenic miRNA cluster, located in a ~60-kb interval between the genes encoding nuclear respiratory factor-1 (Nrf1) and ubiquitin-conjugating enzyme E2H (Ube2h) on mouse chr6qA3.3 (1). To start to uncover the genomic structure of the miR- 183/96/182 gene, we first studied genomic features around miR-183/96/182 in the UCSC genome browser (http://genome.UCSC.edu/), and identified two CpG islands 3.4-6.5 kb 5’ of pre-miR-183, the most 5’ miRNA of the cluster (Fig. 1A; Fig. S1 and Seq. S1). A cDNA clone, AK044220, located at 3.2-4.6 kb 5’ to pre-miR-183, encompasses the second CpG island (Fig. 1A; Fig. S1). We hypothesized that this cDNA clone was derived from 5’ exon(s) of the primary transcript of the miR-183/96/182 gene, as CpG islands are often associated with promoters (2). Supporting this hypothesis, multiple expressed sequences detected by gene-trap clones, including clone D016D06 (3, 4), were co-localized with the cDNA clone AK044220 (Fig. 1A; Fig. S1). Clone D016D06, deposited by the German GeneTrap Consortium (GGTC) (http://tikus.gsf.de) (3, 4), was derived from insertion of a retroviral construct, rFlpROSAβgeo in 129S2 ES cells (Fig. 1A and C). The rFlpROSAβgeo construct carries a promoterless reporter gene, the β−geo cassette - an in-frame fusion of the β-galactosidase and neomycin resistance (Neor) gene (5), with a splicing acceptor (SA) immediately upstream, and a polyA signal downstream of the β−geo cassette (Fig. -

Supplementary Data
SUPPLEMENTARY DATA A cyclin D1-dependent transcriptional program predicts clinical outcome in mantle cell lymphoma Santiago Demajo et al. 1 SUPPLEMENTARY DATA INDEX Supplementary Methods p. 3 Supplementary References p. 8 Supplementary Tables (S1 to S5) p. 9 Supplementary Figures (S1 to S15) p. 17 2 SUPPLEMENTARY METHODS Western blot, immunoprecipitation, and qRT-PCR Western blot (WB) analysis was performed as previously described (1), using cyclin D1 (Santa Cruz Biotechnology, sc-753, RRID:AB_2070433) and tubulin (Sigma-Aldrich, T5168, RRID:AB_477579) antibodies. Co-immunoprecipitation assays were performed as described before (2), using cyclin D1 antibody (Santa Cruz Biotechnology, sc-8396, RRID:AB_627344) or control IgG (Santa Cruz Biotechnology, sc-2025, RRID:AB_737182) followed by protein G- magnetic beads (Invitrogen) incubation and elution with Glycine 100mM pH=2.5. Co-IP experiments were performed within five weeks after cell thawing. Cyclin D1 (Santa Cruz Biotechnology, sc-753), E2F4 (Bethyl, A302-134A, RRID:AB_1720353), FOXM1 (Santa Cruz Biotechnology, sc-502, RRID:AB_631523), and CBP (Santa Cruz Biotechnology, sc-7300, RRID:AB_626817) antibodies were used for WB detection. In figure 1A and supplementary figure S2A, the same blot was probed with cyclin D1 and tubulin antibodies by cutting the membrane. In figure 2H, cyclin D1 and CBP blots correspond to the same membrane while E2F4 and FOXM1 blots correspond to an independent membrane. Image acquisition was performed with ImageQuant LAS 4000 mini (GE Healthcare). Image processing and quantification were performed with Multi Gauge software (Fujifilm). For qRT-PCR analysis, cDNA was generated from 1 µg RNA with qScript cDNA Synthesis kit (Quantabio). qRT–PCR reaction was performed using SYBR green (Roche). -

The Centriole Protein CEP76 Negatively Regulates PLK1 Activity in the Cytoplasm for Proper Mitotic Progression
© 2020. Published by The Company of Biologists Ltd | Journal of Cell Science (2020) 133, jcs241281. doi:10.1242/jcs.241281 RESEARCH ARTICLE The centriole protein CEP76 negatively regulates PLK1 activity in the cytoplasm for proper mitotic progression Yutaka Takeda, Kaho Yamazaki, Kaho Hashimoto, Koki Watanabe, Takumi Chinen*,‡ and Daiju Kitagawa*,‡ ABSTRACT are important for accurate mitotic progression (Golsteyn, 1995; Polo-like kinase 1 (PLK1) dynamically changes its localization and Schmucker and Sumara, 2014). Several factors regulate PLK1 plays important roles in proper mitotic progression. In particular, strict localization during mitosis. PLK1 is recruited to centrosomes in a control of cytoplasmic PLK1 is needed to prevent mitotic defects. CDK1-dependent manner (Lee et al., 2014). The initial recruitment However, the regulation of cytoplasmic PLK1 is not fully understood. In of PLK1 to kinetochores is mediated by its interaction with PBIP1 this study, we show that CEP76, a centriolar protein, physically (also known as CENPU; Kang et al., 2006). During anaphase, PLK1 interacts with PLK1 and tightly controls the activation of cytoplasmic binds to PRC1 and changes its localization to the midbody (Hu et al., PLK1 during mitosis in human cells. We found that removal of 2012). In addition, strict control of PLK1 in the cytoplasm is needed centrosomes induced ectopic aggregation of PLK1, which is highly to prevent its aggregation and mitotic defects (Mukhopadhyay and phosphorylated, in the cytoplasm during mitosis. Importantly, a Dasso, 2010; Schmit et al., 2012; Zhao et al., 2016). However, the targeted RNAi screen revealed that depletion of CEP76 resulted in a detailed mechanisms through which the localization, expression and similar phenotype. -

Molecular Genetics of Microcephaly Primary Hereditary: an Overview
brain sciences Review Molecular Genetics of Microcephaly Primary Hereditary: An Overview Nikistratos Siskos † , Electra Stylianopoulou †, Georgios Skavdis and Maria E. Grigoriou * Department of Molecular Biology & Genetics, Democritus University of Thrace, 68100 Alexandroupolis, Greece; [email protected] (N.S.); [email protected] (E.S.); [email protected] (G.S.) * Correspondence: [email protected] † Equal contribution. Abstract: MicroCephaly Primary Hereditary (MCPH) is a rare congenital neurodevelopmental disorder characterized by a significant reduction of the occipitofrontal head circumference and mild to moderate mental disability. Patients have small brains, though with overall normal architecture; therefore, studying MCPH can reveal not only the pathological mechanisms leading to this condition, but also the mechanisms operating during normal development. MCPH is genetically heterogeneous, with 27 genes listed so far in the Online Mendelian Inheritance in Man (OMIM) database. In this review, we discuss the role of MCPH proteins and delineate the molecular mechanisms and common pathways in which they participate. Keywords: microcephaly; MCPH; MCPH1–MCPH27; molecular genetics; cell cycle 1. Introduction Citation: Siskos, N.; Stylianopoulou, Microcephaly, from the Greek word µικρoκεϕαλi´α (mikrokephalia), meaning small E.; Skavdis, G.; Grigoriou, M.E. head, is a term used to describe a cranium with reduction of the occipitofrontal head circum- Molecular Genetics of Microcephaly ference equal, or more that teo standard deviations -

Centrosome Impairment Causes DNA Replication Stress Through MLK3
bioRxiv preprint doi: https://doi.org/10.1101/2020.01.09.898684; this version posted January 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Centrosome impairment causes DNA replication stress through MLK3/MK2 signaling and R-loop formation Zainab Tayeh 1, Kim Stegmann 1, Antonia Kleeberg 1, Mascha Friedrich 1, Josephine Ann Mun Yee Choo 1, Bernd Wollnik 2, and Matthias Dobbelstein 1* 1) Institute of Molecular Oncology, Göttingen Center of Molecular Biosciences (GZMB), University Medical Center Göttingen, Göttingen, Germany 2) Institute of Human Genetics, University Medical Center Göttingen, Göttingen, Germany *Lead Contact. Correspondence and requests for materials should be addressed to M. D. (e-mail: [email protected]; ORCID 0000-0001-5052-3967) Running title: Centrosome integrity supports DNA replication Key words: Centrosome, CEP152, CCP110, SASS6, CEP152, Polo-like kinase 4 (PLK4), DNA replication, DNA fiber assays, R-loops, MLK3, MK2 alias MAPKAPK2, Seckel syndrome, microcephaly. Highlights: • Centrosome defects cause replication stress independent of mitosis. • MLK3, p38 and MK2 (alias MAPKAPK2) are signalling between centrosome defects and DNA replication stress through R-loop formation. • Patient-derived cells with defective centrosomes display replication stress, whereas inhibition of MK2 restores their DNA replication fork progression and proliferation. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.01.09.898684; this version posted January 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Is Cep70, a Centrosomal Protein with New Roles in Breast Cancer Dissemination and Metastasis, a Facilitator of Epithelial-Mesenchymal Transition?
Is Cep70, a centrosomal protein with new roles in breast cancer dissemination and metastasis, a facilitator of epithelial-mesenchymal transition? Pedro A. Lazo 1,2 1 Experimental Therapeutics and Translational Oncology Program, Instituto de Biología Molecular y Celular del Cáncer, Consejo Superior de Investigaciones Científicas (CSIC), Universidad de Salamanca, Salamanca, Spain 2 Instituto de Investigación Biomédica de Salamanca (IBSAL), Hospital Universitario de Salamanca, Salamanca, Spain Running title: Cep70 and EMT Disclosures: None declared. Contact address: [email protected] 1 Introduction Microtubules are driving mechanisms of chromosomes, intracellular organelle movement, and cell shape and motility. Microtubules are organized on centrosomes, which are assembled in microtubule-organizing centers (MTOC). In mitosis there is no nuclear envelope and centromeres associated to microtubules are mainly involved in chromosome redistribution into daughter cells. In differentiated cells the microtubule- organizing centers are dispersed in the cytoplasm (non centrosomal (ncMTOC)) and interact with the minus end of microtubules through γ-tubulin. 1 However, it is not known if the microtubule contribution to tumor biology is only by facilitating tumor aneuploidy. During tumor dissemination, a process not linked to cell division, important changes take place in cell shape and motility. Microtubules are very dynamic because of their inherent structural instability, and this plasticity facilitates their reorganization during the epithelial-mesenchymal -

Centriole Overduplication Is the Predominant Mechanism Leading to Centrosome Amplification in Melanoma
Published OnlineFirst January 12, 2018; DOI: 10.1158/1541-7786.MCR-17-0197 Oncogenes and Tumor Suppressors Molecular Cancer Research Centriole Overduplication is the Predominant Mechanism Leading to Centrosome Amplification in Melanoma Ryan A. Denu1,2, Maria Shabbir3, Minakshi Nihal3, Chandra K. Singh3, B. Jack Longley3,4,5, Mark E. Burkard2,4, and Nihal Ahmad3,4,5 Abstract Centrosome amplification (CA) is common in cancer and can evaluated. PLK4 is significantly overexpressed in melanoma com- arise by centriole overduplication or by cell doubling events, pared with benign nevi and in a panel of human melanoma cell including the failure of cell division and cell–cell fusion. To assess lines (A375, Hs294T, G361, WM35, WM115, 451Lu, and SK-MEL- the relative contributions of these two mechanisms, the number of 28) compared with normal human melanocytes. Interestingly, centrosomes with mature/mother centrioles was examined by although PLK4 expression did not correlate with CA in most cases, immunofluorescence in a tissue microarray of human melanomas treatment of melanoma cells with a selective small-molecule PLK4 and benign nevi (n ¼ 79 and 17, respectively). The centrosomal inhibitor (centrinone B) significantly decreased cell proliferation. protein 170 (CEP170) was used to identify centrosomes with The antiproliferative effects of centrinone B were also accompa- mature centrioles; this is expected to be present in most centro- nied by induction of apoptosis. somes with cell doubling, but on fewer centrosomes with over- duplication. Using this method, it was determined that the major- Implications: This study demonstrates that centriole overdupli- ity of CA in melanoma can be attributed to centriole overduplica- cation is the predominant mechanism leading to centrosome tion rather than cell doubling events. -
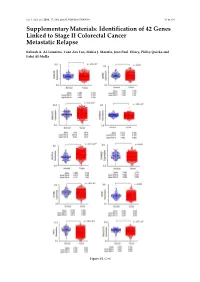
Identification of 42 Genes Linked to Stage II Colorectal Cancer Metastatic Relapse
Int. J. Mol. Sci. 2016, 17, 598; doi:10.3390/ijms17040598 S1 of S16 Supplementary Materials: Identification of 42 Genes Linked to Stage II Colorectal Cancer Metastatic Relapse Rabeah A. Al-Temaimi, Tuan Zea Tan, Makia J. Marafie, Jean Paul Thiery, Philip Quirke and Fahd Al-Mulla Figure S1. Cont. Int. J. Mol. Sci. 2016, 17, 598; doi:10.3390/ijms17040598 S2 of S16 Figure S1. Mean expression levels of fourteen genes of significant association with CRC DFS and OS that are differentially expressed in normal colon compared to CRC tissues. Each dot represents a sample. Table S1. Copy number aberrations associated with poor disease-free survival and metastasis in early stage II CRC as predicted by STAC and SPPS combined methodologies with resident gene symbols. CN stands for copy number, whereas CNV is copy number variation. Region Cytoband % of CNV Count of Region Event Gene Symbols Length Location Overlap Genes chr1:113,025,076–113,199,133 174,057 p13.2 CN Loss 0.0 2 AKR7A2P1, SLC16A1 chr1:141,465,960–141,822,265 356,305 q12–q21.1 CN Gain 95.9 1 SRGAP2B MIR5087, LOC10013000 0, FLJ39739, LOC10028679 3, PPIAL4G, PPIAL4A, NBPF14, chr1:144,911,564–146,242,907 1,331,343 q21.1 CN Gain 99.6 16 NBPF15, NBPF16, PPIAL4E, NBPF16, PPIAL4D, PPIAL4F, LOC645166, LOC388692, FCGR1C chr1:177,209,428–177,226,812 17,384 q25.3 CN Gain 0.0 0 chr1:197,652,888–197,676,831 23,943 q32.1 CN Gain 0.0 1 KIF21B chr1:201,015,278–201,033,308 18,030 q32.1 CN Gain 0.0 1 PLEKHA6 chr1:201,289,154–201,298,247 9093 q32.1 CN Gain 0.0 0 chr1:216,820,186–217,043,421 223,235 q41 CN -

The Transformation of the Centrosome Into the Basal Body: Similarities and Dissimilarities Between Somatic and Male Germ Cells and Their Relevance for Male Fertility
cells Review The Transformation of the Centrosome into the Basal Body: Similarities and Dissimilarities between Somatic and Male Germ Cells and Their Relevance for Male Fertility Constanza Tapia Contreras and Sigrid Hoyer-Fender * Göttingen Center of Molecular Biosciences, Johann-Friedrich-Blumenbach Institute for Zoology and Anthropology-Developmental Biology, Faculty of Biology and Psychology, Georg-August University of Göttingen, 37077 Göttingen, Germany; [email protected] * Correspondence: [email protected] Abstract: The sperm flagellum is essential for the transport of the genetic material toward the oocyte and thus the transmission of the genetic information to the next generation. During the haploid phase of spermatogenesis, i.e., spermiogenesis, a morphological and molecular restructuring of the male germ cell, the round spermatid, takes place that includes the silencing and compaction of the nucleus, the formation of the acrosomal vesicle from the Golgi apparatus, the formation of the sperm tail, and, finally, the shedding of excessive cytoplasm. Sperm tail formation starts in the round spermatid stage when the pair of centrioles moves toward the posterior pole of the nucleus. The sperm tail, eventually, becomes located opposed to the acrosomal vesicle, which develops at the anterior pole of the nucleus. The centriole pair tightly attaches to the nucleus, forming a nuclear membrane indentation. An Citation: Tapia Contreras, C.; articular structure is formed around the centriole pair known as the connecting piece, situated in the Hoyer-Fender, S. The Transformation neck region and linking the sperm head to the tail, also named the head-to-tail coupling apparatus or, of the Centrosome into the Basal in short, HTCA.