Biological Fronts in the Strait of Georgia, British Columbia, and Their Relation to Recent Measurements of Primary Productivity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ecosystem Status and Trends Report for the Strait of Georgia Ecozone
C S A S S C C S Canadian Science Advisory Secretariat Secrétariat canadien de consultation scientifique Research Document 2010/010 Document de recherche 2010/010 Ecosystem Status and Trends Report Rapport de l’état des écosystèmes et for the Strait of Georgia Ecozone des tendances pour l’écozone du détroit de Georgie Sophia C. Johannessen and Bruce McCarter Fisheries and Oceans Canada, Institute of Ocean Sciences 9860 W. Saanich Rd. P.O. Box 6000, Sidney, B.C. V8L 4B2 This series documents the scientific basis for the La présente série documente les fondements evaluation of aquatic resources and ecosystems scientifiques des évaluations des ressources et in Canada. As such, it addresses the issues of des écosystèmes aquatiques du Canada. Elle the day in the time frames required and the traite des problèmes courants selon les documents it contains are not intended as échéanciers dictés. Les documents qu’elle definitive statements on the subjects addressed contient ne doivent pas être considérés comme but rather as progress reports on ongoing des énoncés définitifs sur les sujets traités, mais investigations. plutôt comme des rapports d’étape sur les études en cours. Research documents are produced in the official Les documents de recherche sont publiés dans language in which they are provided to the la langue officielle utilisée dans le manuscrit Secretariat. envoyé au Secrétariat. This document is available on the Internet at: Ce document est disponible sur l’Internet à: http://www.dfo-mpo.gc.ca/csas/ ISSN 1499-3848 (Printed / Imprimé) ISSN 1919-5044 (Online / En ligne) © Her Majesty the Queen in Right of Canada, 2010 © Sa Majesté la Reine du Chef du Canada, 2010 TABLE OF CONTENTS Highlights 1 Drivers of change 2 Status and trends indicators 2 1. -

Spring Bloom in the Central Strait of Georgia: Interactions of River Discharge, Winds and Grazing
MARINE ECOLOGY PROGRESS SERIES Vol. 138: 255-263, 1996 Published July 25 Mar Ecol Prog Ser I l Spring bloom in the central Strait of Georgia: interactions of river discharge, winds and grazing Kedong yinl,*,Paul J. Harrisonl, Robert H. Goldblattl, Richard J. Beamish2 'Department of Oceanography, University of British Columbia, Vancouver, British Columbia, Canada V6T 124 'pacific Biological Station, Department of Fisheries and Oceans, Nanaimo, British Columbia, Canada V9R 5K6 ABSTRACT: A 3 wk cruise was conducted to investigate how the dynamics of nutrients and plankton biomass and production are coupled with the Fraser River discharge and a wind event in the Strait of Georgia estuary (B.C.,Canada). The spring bloom was underway in late March and early Apnl, 1991. in the Strait of Georgia estuary. The magnitude of the bloom was greater near the river mouth, indicat- ing an earher onset of the spring bloom there. A week-long wind event (wind speed >4 m S-') occurred during April 3-10 The spring bloom was interrupted, with phytoplankton biomass and production being reduced and No3 in the surface mixing layer increasing at the end of the wind event. Five days after the lvind event (on April 15),NO3 concentrations were lower than they had been at the end of the wind event, Indicating a utilization of NO3 during April 10-14. However, the utilized NO3 did not show up in phytoplankton blomass and production, which were lower than they had been at the end (April 9) of the wind event. During the next 4 d, April 15-18, phytoplankton biomass and production gradu- ally increased, and No3 concentrations in the water column decreased slowly, indicating a slow re- covery of the spring bloom Zooplankton data indicated that grazing pressure had prevented rapid accumulation of phytoplankton biomass and rapid utilization of NO3 after the wind event and during these 4 d. -

A Nitrogen Budget for the Strait of Georgia, British Columbia, with Emphasis on Particulate Nitrogen and Dissolved Inorganic Nitrogen
Biogeosciences, 10, 7179–7194, 2013 Open Access www.biogeosciences.net/10/7179/2013/ doi:10.5194/bg-10-7179-2013 Biogeosciences © Author(s) 2013. CC Attribution 3.0 License. A nitrogen budget for the Strait of Georgia, British Columbia, with emphasis on particulate nitrogen and dissolved inorganic nitrogen J. N. Sutton1,2, S. C. Johannessen1, and R. W. Macdonald1 1Institute of Ocean Sciences, Fisheries and Oceans Canada, 9860 West Saanich Road, P.O. Box 6000, Sidney, British Columbia, V8L 4B2, Canada 2Department of Earth and Planetary Science, University of California, Berkeley, California, 94720, USA Correspondence to: J. N. Sutton ([email protected]) Received: 6 March 2013 – Published in Biogeosciences Discuss.: 23 April 2013 Revised: 29 September 2013 – Accepted: 10 October 2013 – Published: 12 November 2013 Abstract. Balanced budgets for dissolved inorganic N (DIN) 1 Introduction and particulate N (PN) were constructed for the Strait of Georgia (SoG), a semi-enclosed coastal sea off the west coast of British Columbia, Canada. The dominant control on the The nitrogen (N) cycle is a crucial underpinning of marine N budget is the advection of DIN into and out of the SoG biological productivity (Gruber and Galloway, 2008). Dur- via Haro Strait. The annual influx of DIN by advection from ing the past 150 yr, the global N cycle has been dramati- the Pacific Ocean is 29 990 (±19 500) Mmol yr−1. The DIN cally changed by human activities that have loaded reactive N flux advected out of the SoG is 24 300 (±15 500) Mmol yr−1. into ecosystems in amounts that rival natural sources (Rabal- Most of the DIN that enters the SoG (∼ 23 400 Mmol yr−1) ais, 2002; Galloway et al., 2004). -

Psc Draft1 Bc
138°W 136°W 134°W 132°W 130°W 128°W 126°W 124°W 122°W 120°W 118°W N ° 2 6 N ° 2 DR A F T To navigate to PSC Domain 6 1/26/07 maps, click on the legend or on the label on the map. Domain 3: British Columbia R N ° k 0 6 e PSC Region N s ° l Y ukon T 0 e 6 A rritory COBC - Coastal British Columbia Briti sh Columbia FRTH - Fraser R - Thompson R GST - Georgia Strait . JNST - Johnstone Strait R ku NASK - Nass R - Skeena R Ta N QCI - Queen Charlotte Islands ° 8 5 TRAN N TRAN - Transboundary Rivers in Canada ° 8 r 5 ive R WCVI - Western Vancouver Island e in r !. City/Town ik t e v S i Major River R t u k Scale = 1:6,750,000 Is P Miles N ° 0 30 60 120 180 January 2007 6 A B 5 N ° r 6 i 5 C t i s . A h R Alaska l I b C s e F s o a NASK r l N t u a r m I e v S i C tu b R a i rt a N a ° Prince Rupert en!. R 4 ke 5 !. S Terrace iv N e ° r 4 O F 5 !. ras er C H Prince George R e iv c e QCI a r t E e . r R S ate t kw r lac Quesnel A a B !. it D e an R. N C F N COBC h FRTH ° i r 2 lc a 5 o N s ti ° e n !. -

Marine Recreation in the Desolation Sound Region of British Columbia
MARINE RECREATION IN THE DESOLATION SOUND REGION OF BRITISH COLUMBIA by William Harold Wolferstan B.Sc., University of British Columbia, 1964 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS in the Department of Geography @ WILLIAM HAROLD WOLFERSTAN 1971 SIMON FRASER UNIVERSITY December, 1971 Name : William Harold Wolf erstan Degree : Master of Arts Title of Thesis : Marine Recreation in the Desolation Sound Area of British Columbia Examining Committee : Chairman : Mar tin C . Kellman Frank F . Cunningham1 Senior Supervisor Robert Ahrens Director, Parks Planning Branch Department of Recreation and Conservation, British .Columbia ABSTRACT The increase of recreation boating along the British Columbia coast is straining the relationship between the boater and his environment. This thesis describes the nature of this increase, incorporating those qualities of the marine environment which either contribute to or detract from the recreational boating experience. A questionnaire was used to determine the interests and activities of boaters in the Desolation Sound region. From the responses, two major dichotomies became apparent: the relationship between the most frequented areas to those considered the most attractive and the desire for natural wilderness environments as opposed to artificial, service- facility ones. This thesis will also show that the most valued areas are those F- which are the least disturbed. Consequently, future planning must protect the natural environment. Any development, that fails to consider the long term interests of the boater and other resource users, should be curtailed in those areas of greatest recreation value. iii EASY WILDERNESS . Many of us wish we could do it, this 'retreat to nature'. -

The Damnation of a Dam : the High Ross Dam Controversy
THE DAMYIATION OF A DAM: TIIE HIGH ROSS DAM CONTROVERSY TERRY ALLAN SIblMONS A. B., University of California, Santa Cruz, 1968 A THESIS SUBIUTTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS in the Department of Geography SIMON FRASER UNIVERSITY May 1974 All rights reserved. This thesis may not b? reproduced in whole or in part, by photocopy or other means, without permission of the author. APPROVAL Name: Terry Allan Simmons Degree: Master of Arts Title of Thesis: The Damnation of a Dam: The High Ross Dam Controversy Examining Committee: Chairman: F. F. Cunningham 4 E.. Gibson Seni Supervisor / /( L. J. Evendon / I. K. Fox ernal Examiner Professor School of Community and Regional Planning University of British Columbia PARTIAL COPYRIGHT LICENSE I hereby grant to Simon Fraser University rhe righc to lcnd my thesis or dissertation (the title of which is shown below) to users of the Simon Fraser University Library, and to make partial or single copies only for such users or in response to a request from the library of any other university, or other educational institution, on its own behalf or for one of its users. I further agree that permission for multiple copying of this thesis for scholarly purposes may be granted by me or the Dean of Graduate Studies. It is understood that copying or publication of this thesis for financial gain shall not be allowed ' without my written permission. Title of' ~hesis /mqqmkm: The Damnation nf a nam. ~m -Author: / " (signature ) Terrv A. S.imrnonze (name ) July 22, 1974 (date) ABSTRACT In 1967, after nearly fifty years of preparation, inter- national negotiations concerning the construction of the High Ross Dan1 on the Skagit River were concluded between the Province of British Columbia and the City of Seattle. -
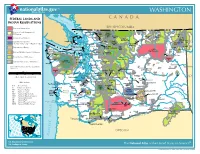
WASHINGTON Where We Are
nationalatlas.gov TM WASHINGTON Where We Are FEDERAL LANDS AND CANADA Str ait INDIAN RESERVATIONS of Ge org ia BRITISH COLUMBIA Bureau of Indian Affairs Blaine Mount Baker Bureau of Land Management / Oroville Helicopter Training Mount North Wilderness Area Baker Lummi IR Cascades Colville Colville NF NP Ross Lake Okanogan San Juan Islands National Forest R NF Bureau of Reclamation Bellingham NRA NF Cusick NWR R Okanogan a S Conconully Republic i Survival tr National Forest Lake b Training Kaniksu Department of Defense ai San Juan Island Mount Baker North t o Anacortes Skagit NRA m Site NF f NHP Cascades Little Pend Oreille (includes Army Corps of Engineers lakes) Jua Swinomish IR Conconully u n NP l NWR de Reservoir Omak Makah F o uca Whidbey Island NAS Mount Vernon Mount Baker Lake Kalispel IR Department of Energy IR Helicopter Training Area Chelan C Colville Flattery Rocks NWR Naval NRA Colville NF Kaniksu Ebey's Landing NHR Reservation Ozette IR Lower Elwha IR Indian Reservation NF Fish and Wildlife Service / Wilderness Lakewood Mount Baker Newport Olympic Port Angeles Dungeness Olympic Protection Island National Forest Lake Roosevelt NP NWR Tulalip IR NF NWR National Recreation Area Forest Service / Wilderness Everett Forks Olympic NF Wenatchee Grand Coulee Spokane Quileute IR National Forest Olympic Port Gamble IR IR National Park Service / Wilderness National Park d Lynnwood Snoqualmie Port Madison IR n Chelan Hoh IR u NF Banks Wilbur o S Lake Spokane t Seattle Some small sites are not shown, especially in R e Opportunity t g ul Bremerton Leavenworth Coulee City u Bellevue urban areas. -

The Fraser River Plume
new horizons centrepiece The Fraser River Plume Martin Evans uses the example of a dramatic ocean phenomenon to show how all the elements of geography interact to shape the physical and human landscape The water cycle and Human settlement GeographyReviewExtras the glacial history The flat oceanside land of the delta and the rich marine food resources have led to You can download a pdf of The Fraser River drains the interior of a long history of settlement by Canadian this spread to print as a poster at: British Columbia on Canada’s west coast www.hoddereducation.co.uk/ indigenous peoples. The first settlement with a catchment area over ten times the here was up to 9,000 years ago. This was geographyreviewextras 2 area of Wales (220,000 km ). In the spring an important trading area for aboriginal the melting of snow and glacial ice in the peoples and is now the site of Vancouver mountains of British Columbia sends a pulse (population 2.4 million) which is a major What is the Fraser of meltwater down the Fraser. port city, trading around US$200 billion in River Plume? The Fraser valley contains thick deposits goods annually. of glacial sediments deposited at the end If you take the Tsawwassen ferry from of the last ice age and mobilisation of some Vancouver to Vancouver Island during of this material in the spring flood produces the summer you may find yourself sailing high sediment concentrations in the river How geographical through a dramatic shift in the colour of water. Deposition of these sediments The Fraser Delta and the city the ocean surface. -
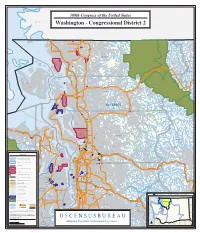
U N S U U S E U R a C S
108th Congress of the United States C A N A D A CANADA Blaine Nooksack Trust Land Sumas Semiahmoo Bay 9 e Drayton t R Harbor t StRte 546 (Badger Rd) S Lynden Birch Bay Peaceful N o o Valley ks ac Nooksack Trust Land k R iv er Birch Bay Nooksack StRte 548 (Blaine Rd) Custer Everson Kendall Maple Falls StRte 548 (Grandview Rd) StRte 544 (Pole Rd) StRte 9 Strait of Georgia Glacier Nooksack Lake Terrell Trust Land Nooksack Trust Land ) y w Ferndale 2 H 4 r 5 e e k t a R t B Deming t S North Cascades Natl Pk n WHATCOM u o Ross (M Lake Nooksack Res StRte 542 Nooksack Trust Marietta- Land Alderwood OKANOGAN 5 Nooksack Trust Land Echo Bay Lummi Bellingham Res Boundary Pass President Channel Geneva Baker Lake Hales Passage StRte 9 (Valley Hwy) Sudden Hwy) es Bellingham cad Valley Acme as Bay 20 (C Ross Lake Natl Rec Area te R S t Cowlitz Bay Lake DISTRICT Chuckanut Whatcom Bay 5 New Channel Lake Samish S t West H w Alger y 1 Sound 1 Haro Strait ( C h East u S c tH k w Sound a y n Samish Bay u 2 Harney t 0 D Channel B ( San Juan Channel r) S e Lake Shannon t l R l i d n Samish TDSA 2 g 0 h ) Rosario Strait SAN JUAN a r m e Upright Upper v Rosario Strait i Channel C Skagit R h Edison a Res it Friday Lopez Sound n g nel a k Harbor S Friday Upper Skagit Res Hamilton Concrete North Cascades Natl Pk Lyman Harbor Guemes Channel StHwy 20 Ska Marblemount Sedro-Woolley git R iver Fidalgo Griffin Bay Bay Anacortes SKAGIT Rockport Padilla Bay View Bay Burrows Burlington Bay 0 StHwy 2 StH Clear Lake P w y Similk u (Memoria 5 StHwy 538 g l 36 Bay e H w t (College -

Five Easy Pieces on the Strait of Georgia – Reflections on the Historical Geography of the North Salish Sea
FIVE EASY PIECES ON THE STRAIT OF GEORGIA – REFLECTIONS ON THE HISTORICAL GEOGRAPHY OF THE NORTH SALISH SEA by HOWARD MACDONALD STEWART B.A., Simon Fraser University, 1975 M.Sc., York University, 1980 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE AND POSTDOCTORAL STUDIES (Geography) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) October 2014 © Howard Macdonald Stewart, 2014 Abstract This study presents five parallel, interwoven histories of evolving relations between humans and the rest of nature around the Strait of Georgia or North Salish Sea between the 1850s and the 1980s. Together they comprise a complex but coherent portrait of Canada’s most heavily populated coastal zone. Home to about 10% of Canada’s contemporary population, the region defined by this inland sea has been greatly influenced by its relations with the Strait, which is itself the focus of a number of escalating struggles between stakeholders. This study was motivated by a conviction that understanding this region and the sea at the centre of it, the struggles and their stakeholders, requires understanding of at least these five key elements of the Strait’s modern history. Drawing on a range of archival and secondary sources, the study depicts the Strait in relation to human movement, the Strait as a locus for colonial dispossession of indigenous people, the Strait as a multi-faceted resource mine, the Strait as a valuable waste dump and the Strait as a place for recreation / re-creation. Each of these five dimensions of the Strait’s history was most prominent at a different point in the overall period considered and constantly changing relations among the five narratives are an important focus of the analysis. -

Marine Birds
OCEAN WATCH | Howe Sound Edition SPECIES AND HABITATS Marine Birds AUTHORS Karl Ricker,1 Geologist, Biologist, Glaciologist, What is happening with Mountaineer, Citizen Scientist, Whistler Bob Turner, Geoscientist and Citizen Scientist, Bowen Island, Howe Sound marine birds? REVIEWER If you are out on the waters of Howe Sound, you are more likely to see and Rob Butler, Pacific Wildlife Foundation hear marine birds than any other wildlife. Marine birds animate Howe Sound with sounds of gulls, roosting cormorants at Horseshoe Bay, and great rafts of scoters and goldeneye that provide a magnificent shoreline spectacle dur- ing the winter months. Because marine birds are highly visible, changes in their populations are easier to observe than other species and they’ve be- come important indicators of environmental stress.2 But recent reviews of marine birds throughout the Strait of Georgia and Salish Sea have identified long term declines in a number of species that raise serious concerns.3,4 A recent assessment compares changes in marine bird counts in Howe Sound to changes noted in the Strait of Georgia and finds that changes in Howe Sound winter bird counts tend to parallel, with a few exceptions, the trends in nearby coastal marine birds as documented for the Strait of Georgia.5 Most of what we know about marine birds in Howe Sound comes from the observations of volunteers over many years. Volunteers conduct an- nual Christmas bird counts and monthly counts at several locations within Howe Sound. For example, in December 2015 and January -

Marine Atlas of Pacific Canada 142°W 140°W 138°W 136°W 134°W 132°W 130°W 128°W 126°W 124°W 122°W
Physical Representation – Ecosections description data sources This atlas page illustrates the 12 marine ecosections delineated by the Province of British Columbia in 2000. Ecosections are recognized by • Province of British Columbia a hierarchical ecological mapping system called the British Columbia Marine Ecological Classification (BCMEC) and defined according to physical, oceanographic and biological characteristics. They are a very useful delineation in a physical sense in that they have been mapped with data resolution a focus on identifying physical differences that will tend to affect species distributions within a bio-geographic region. • Ecosections are mapped at small scales (1:250,000) for resource emphasis and area planning. date of analysis • 2000 (published in 2002) reviewers • Kim Conway, Natural Resources Canada • Zach Ferdana, The Nature Conservancy reviewer comments PHOTO: CHARLIEPHOTO: SHORT PHOTO: ROBERT SIMON ROBERT PHOTO: • None provided. caveats of use • The Province of BC is not liable for errors in the data or inappropriate usage of the data. • Recommended date of expiry for use of these data in a marine planning context: None provided. map, feature data and metadata access • Visit www.bcmca.ca/data for more information. references • For more detailed information on the Marine Ecosections of British Columbia see: Ministry of Sustainable Resource Management (MSRM), Decision Support Services Branch. British Columbia Marine Ecological Classification Marine Ecosections and Ecounits, Version 2.0. Report prepared for