24 Lupus Anticoagulants: Mechanistic and Diagnostic Considerations Jef M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Domino Effect in a Patient with Epstein-Barr Virus Infection and Autoimmunity: a Case Report Case Report Appointments Scheduled
International Journal of Case Report Clinical Rheumatology Domino effect in a patient with Epstein- Barr Virus infection and autoimmunity: A case report The link between autoimmune diseases and viral infections has been characterized, but specific Margarite Matossian, Carrie mechanisms behind this association remain a current area of investigation. Whether viral infections Crook, Alec Goldberg, Anna trigger or unmask autoimmunity, or if the pathologies occur concurrently, is not yet completely Stathopoulos, Justin Tien, understood. Specifically, Epstein - Barr virus (EBV) is implicated in several autoimmune disorders, Sheela Sheth, Osaid Saqqa & including Systemic Lupus Erythematosus (SLE). It is hypothesized that common immunologic Christopher Dale Shamburger* pathways are activated in the two pathologic states. This case report is an example of this confusing Department of Medicine, Tulane University presentation, and the importance of recognizing the association between autoimmunity and viral Health Sciences Center, New Orleans LA infections. This patient presented with symptoms concerning for SLE and hepatic autoimmunity 70115, USA with serology suggesting a recent infection with EBV. Given this complicated presentation, it is *Author for correspondence: difficult to determine which disease state presented first in patients with evidence of both SLE and EBV infection and whether this information is clinically relevant for ongoing treatment and [email protected] monitoring. Here, we provide an in-depth discussion of current genomic and immunological research that supports the associations amongst these disease pathologies. Introduction by an intricate psychiatric history. Then Autoimmune diseases have increased in we discuss the contribution of infection to frequency in industrialized countries over autoimmune disease states and important recent years [1]. The etiology of autoimmune distinctions between autoimmune diseases diseases, a self-reactive adaptive immune and autoinflammatory responses. -

Pediatric Motor Inflammatory Neuropathy: the Role Of
brain sciences Case Report Pediatric Motor Inflammatory Neuropathy: The Role of Antiphospholipid Antibodies Claudia Brogna 1,2,*, Marco Luigetti 3 , Giulia Norcia 1, Roberta Scalise 1, Gloria Ferrantini 1, Beatrice Berti 1, Domenico M. Romeo 4, Raffaele Manna 5, Eugenio Mercuri 1 and Marika Pane 1 1 Pediatric Neurology and Nemo Clinical Centre, Fondazione Policlinico Universitario A. Gemelli IRCSS, Università Cattolica del Sacro Cuore, 00168 Roma, Italy; [email protected] (G.N.); [email protected] (R.S.); [email protected] (G.F.); [email protected] (B.B.); [email protected] (E.M.); [email protected] (M.P.) 2 Pediatric Neuropsichiatric Unit, ASL, 83100 Avellino, Italy 3 Institute of Neurology, Fondazione Policlinico Universitario A. Gemelli IRCSS, Università Cattolica del sacro Cuore, 00168 Roma, Italy; [email protected] 4 Pediatric Neurology, Fondazione Policlinico Universitario A. Gemelli IRCSS, Università Cattolica del Sacro Cuore, 00168 Roma, Italy; [email protected] 5 Periodic Fevers Research Centre and rare disease. Department of Internal Medicine, Fondazione Policlinico Universitario A. Gemelli IRCSS, Università Cattolica del Sacro Cuore, 00168 Roma, Italy; raff[email protected] * Correspondence: [email protected] Received: 4 February 2020; Accepted: 5 March 2020; Published: 7 March 2020 Abstract: We report the clinical case of a nine-year-old girl who presented with progressive motor neuropathy, revealed via the detection of a higher delay in F-wave recording using digitalized nerve conduction/electromyography. Since the lupus anticoagulant (LAC) positivity, detected using diluted Russell viper venom time (dRVVT), switched to persistent serological anticardiolipin immunoglobulin G (IgG) positivity, a possible non-thrombotic antiphospholipid antibody (aPL)-related clinical manifestation was suspected, and intravenous immunoglobulin treatment (IVIG) was started. -
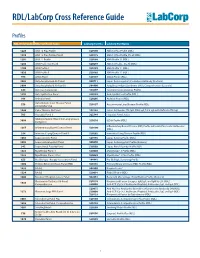
RDL/Labcorp Cross Reference Guide
RDL/LabCorp Cross Reference Guide Profiles RDL Order Code RDL Test/Panel Name LabCorp Test No. LabCorp Test Name 1228 ANA 12 Plus Profile 520180 ANA 12 Plus Profile (RDL) 1230 ANA 12 Plus Profile, Do All 520175 ANA 12 Plus Profile, Do all (RDL) 1201 ANA 12 Profile 520188 ANA Profile 12 (RDL) 1206 ANA Profile 12, Do All 520299 ANA 12 Profile , Do All (RDL) 1100 ANA Profile I 520185 ANA Profile 11 (RDL) 1020 ANA Profile II 520185 ANA Profile 11 (RDL) 994 ANCA Panel 520149 ANCA Profile (RDL) 3003 Antiphospholipid Ab Panel I 500711 Lupus Anticoagulant/Cardiolipin Antibody (Esoterix) 3004 Antiphospholipid Ab Panel II 504400 Antiphospholipid Syndrome (APS), Comprehensive (Esoterix) 644 Anti-Saccharomyces 164657 Saccharomyces cerevisiae Profile 1292 Anti-Synthetase Panel 520193 Anti-Synthetase Profile (RDL) 996 Arthritis Panel 520205 Arthritis Profile (RDL) Autoimmune Liver Disease Panel, 558 520197 Autoimmune Liver Disease Profile (RDL) Comprehensive 1044 Celiac Disease Ab Panel 165142 Celiac Antibodies tTG IgA, EMA IgA, Total IgA with Reflex to tTG IgG 705 Hepatitis Panel II 322744 Hepatitis Panel, Acute ILDdxComplete (Interstitial Lung Disease 3008 520210 ILDdx Profile (RDL) Complete) Inflammatory Bowel Disease (IBD) Profile with Anti-Pancreatic Antibodies 1265 Inflammatory Bowel Disease Panel 520190 (RDL) 354 Interstitial Lung Disease Panel II 520202 Interstitial Lung Disease Profile (RDL) 1065 Lupus Activity Panel 520195 Lupus Activity Profile (RDL) 3002 Lupus Anticoagulant Panel 500070 Lupus Anticoagulant Profile (Esoterix) 342 Lupus Renal -

32 Mechanism of Thrombosis in Antiphospholipid Syndrome: Binding to Platelets Joan-Carles Reverter and Dolors Tàssies
32 Mechanism of Thrombosis in Antiphospholipid Syndrome: Binding to Platelets Joan-Carles Reverter and Dolors Tàssies Introduction Antiphospholipid antibodies (aPL) are related to thrombosis in the antiphospho- lipid syndrome (APS) [1, 2] and numerous pathophysiological mechanisms have been suggested involving cellular effects, plasma coagulation regulatory proteins, and fibrinolysis [3, 4]: aPL may act as blocking agents directly inhibiting antigen enzymatic or co-factor function of hemostasis; may bind fluid-phase antigens of hemostasis involved proteins and then decrease plasma antigen levels by clearance of immune complexes; may form immune complexes with their antigens that may be deposited in blood vessels causing inflammation and tissue injury; may cause dysregulation of antigen–phospholipid binding due to cross-linking of membrane bound antigens; and may trigger cell mediated events by cross-linking of antigen bound to cell surfaces or cell surface receptors [3, 4]. Moreover, several characteris- tics of the aPL, such as the concentration, class/subclass, affinity or charge, and several characteristics of the antigens, as the concentration, size, location or charge, may influence which of the theoretical autoantibody actions will occur in vivo [3]. Among the cellular mechanisms supposed to be involved, platelets have been considered as one of the most promising potential target for circulating aPL that may cause antibody mediated thrombosis as a part of the clinical spectrum of the autoimmune disorder of the APS. In the present chapter we will focus on the inter- actions that involve aPL binding to platelet membrane or platelet membrane bound antigens. Platelets as Target for aPL Platelets play a central role in primary hemostasis involving platelet adhesion to the injured blood vessel wall, followed by platelet activation, granule release, shape change, and rearrangement of the outer membrane phospholipids and proteins, transforming them into a highly efficient procoagulant surface [5]. -

Significant Factors Associated with Lupus Nephritis P Alba, L Bento, M J Cuadrado, Y Karim, M F Tungekar, I Abbs, M a Khamashta, D D’Cruz,Grvhughes
556 EXTENDED REPORT Ann Rheum Dis: first published as 10.1136/ard.62.6.556 on 1 June 2003. Downloaded from Anti-dsDNA, anti-Sm antibodies, and the lupus anticoagulant: significant factors associated with lupus nephritis P Alba, L Bento, M J Cuadrado, Y Karim, M F Tungekar, I Abbs, M A Khamashta, D D’Cruz,GRVHughes ............................................................................................................................. Ann Rheum Dis 2003;62:556–560 Background: Lupus nephritis (LN) is a common manifestation in patients with systemic lupus erythema- tosus (SLE). Autoantibodies and ethnicity have been associated with LN, but the results are controver- sial. Objective: To study the immunological and demographic factors associated with the development of LN. Patients and methods: A retrospective case-control study of 127 patients with biopsy-proven LN, and 206 randomly selected patients with SLE without nephritis as controls was designed. All patients had attended our lupus unit during the past 12 years. Standard methods were used for laboratory testing. Results: Patients with LN were significantly younger than the controls at the time of SLE diagnosis See end of article for (mean (SD) 25.6 (8.8) years v 33.7 (12.5) years; p<0.0001). The proportion of patients of black eth- authors’ affiliations nic origin was significantly higher in the group with nephritis (p=0.02). There were no differences in ....................... sex distribution or duration of follow up. A higher proportion of anti-dsDNA, anti-RNP, anti-Sm, and Correspondence to: lupus anticoagulant (LA) was seen in the group with nephritis (p=0.002; p=0.005; p=0.0001; Dr M J Cuadrado, Lupus p=0.01, respectively). -

Hepatic Manifestations of the Antiphospholipid Syndrome
EDITORIAL Hepatic Manifestations of the Antiphospholipid Syndrome Key words: autoimmune hepatitis, Sjogren's syndrome, Budd- autoimmune hepatitis (7). Examinations prior to liver biopsy Chiari syndrome, nodular regenerative hyperpla- revealed a greatly prolonged APTT,and the patient was found sia to be positive for IgG anticardiolipin antibody and lupus anti- coagulant. However, this patient showedno signs of thrombo- sis or any other symptomssuggestive ofAPS. In this issue, Katayama et al (8) report a case of Sjogren's Antiphospholipid syndrome (APS) is characterized by the syndrome complicated with autoimmune hepatitis and APS. presence of antiphospholipid antibodies (anticardiolipin anti- bodies and/or lupus anticoagulant) in association with symp- See also p 73. toms including venous/arterial thromboses, recurrent fetal loss and thrombocytopenia. Thromboticepisodes mayoccur at any In previously reported cases of autoimmune hepatitis asso- part of the circulation system, hence, any organ may be in- ciated with the presence of antiphospholipid antibodies, anti- volved. As for hepatic involvement, the most well knownmani- nuclear antibody was present, but the presence of anti-SS-A festation ofAPSis Budd-Chiari syndrome, which is caused antibody or sicca symptomshave not been documented. There- mostly by obstruction of hepatic veins or inferior vena cava. fore, this case may possibly be the first documented case of Pelletier et al (1) have reported that of their 22 non-tumourous autoimmune hepatitis with APSand Sjogren's syndrome. Their Budd-Chiari patients, 4 had antiphospholipid antibodies with- patient had autoimmune hepatitis, and Sjogren's syndrome out other causes of hepatic vein thrombosis. Amongthese 4 confirmed by Schirmer's test and sialography. The patient patients, 2 seemed to have primary APS, i.e., no signs of other showed positive for (32-GPI dependent anticardiolipin antibody autoimmunediseases such as SLE. -

Evaluation of Clinical and Laboratory Findings of 147 Patients with Systemic Lupus Erythematosus: the Relationship Between Anti-CCP and Arthritis
J Surg Med. 2019;3(3):227-230. Research article DOI: 10.28982/josam.503850 Araştırma makalesi Evaluation of clinical and laboratory findings of 147 patients with systemic lupus erythematosus: The relationship between anti-CCP and arthritis Sistemik lupus eritematozuslu 147 hastanın klinik ve laboratuvar bulgularının değerlendirilmesi: Anti CCP ile artrit arasındaki ilişkinin incelenmesi Ali Ekin 1, Ayşe Ergüney Çefle 2 1 Department of Internal Medicine, Bingol Abstract State Hospital, Bingol, Turkey Aim: Anti-cyclic citrullinated peptide antibodies (Anti-CCP) is considered as a novel marker in the assessment of 2 Department of Internal Medicine, Division of Rheumatology, Faculty of Medicine, Kocaeli rheumatoid disorders. Some studies have emphasized the importance of anti-CCP in indicating erosive arthropathy in University, Kocaeli, Turkey Systemic Lupus Erythematosus (SLE), like Rheumatoid Arthritis (RA). These studies have reported that the chance of erosive arthritis development is significantly increased in anti-CCP-positive patients. This study aimed to investigate ORCID ID of the author(s) the relationship between anti-CCP and arthritis along with other clinical and laboratory parameters in patients with AE: 0000-0003-3692-1293 SLE. AEÇ: 0000-0002-3273-7969 Methods: A total of 147 SLE patients who had been admitted to Kocaeli University Medical Faculty, Department of Internal Medicine, Division of Rheumatology between January 2001 and October 2015 were included in this retrospective study. SLE diagnosis was verified according to American College of Rheumatology (ACR) and/or The Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) criteria. Patients whose diagnosis was not definite and not having anti-CCP were excluded. Results: Female/male ratio was found as 5.6, and the mean age was calculated as 43.9±11.85 years. -

Northwestern Medicine
How to put in your own Photo: Go to ‘View-Slide Master’ Go to ‘Insert-Picture’ Browse to the image you would like to place. Image should be 1024x768, 1200x900, or other 4:3 aspect ratio Select image and click OK Scale to full screen size if To live with lupus, necessary. Click image, go to ‘Format- we need to know Send to Back’ about lupus. Zineb Aouhab, MD Assistant Professor of Medicine Loyola University Medical Center 1 Division of Rheumatology Where did the word “lupus” come from? • The word ‘lupus’ is derived from the Latin word for “wolf”. • Named in the 13th century by the physician “Rogerius”. • He described the rash as erosive facial lesions as a consequence of a wolf's bite. • The facial rash is also called “Butterfly rash” because it resembles a butterfly shape. 2 What is lupus? • Lupus is an autoimmune disease. • It causes chronic inflammation in the body. • It can affect any organ of the body such as the kidneys, gastrointestinal tract, lungs, heart, and joints. 3 What happens in the body? • In normal people, the immune system forms antibodies to fight against infections. • In people with lupus, the immune system become overactive (like a bad allergic reaction) and produces a different types of antibodies. • These antibodies attack the person’s own organs causing organ damage. 4 Factors to consider in lupus • Lupus usually appears between the age of 15-45. • Lupus is much more common in females compared to males. Ratio 9:1 • Lupus is characterized by episodes of flares (when people feel very ill) and periods of remission (when people feel well and back to baseline). -

A Rare Case of Overlapping Syndrome of ANCA-Associated Glomerulonephritis and Copyright: Systemic Lupus Erythematosus © 2018 Jessica F, Et Al
www.symbiosisonline.org Symbiosis www.symbiosisonlinepublishing.com Case Report Journal of Rheumatology and Arthritic Diseases Open Access A Rare Case of Overlapping Syndrome of ANCA- Associated Glomerulonephritis and Systemic Lupus Erythematosus Jessica Frey1* and Hajra Shah2 1Neurology Resident, Department of Neurology, West Virginia University, 1 Medical Center Drive, Morgantown WV 26505 USA 2Assistant Professor of Medicine, Department of Rheumatology, West Virginia University, 1 Medical Center Drive, Morgantown WV 26505 USA Received: June 15, 2018; Accepted: July 15, 2018; Published: July 23, 2018 *Corresponding author: Jessica Frey, MD, 99 Lakeside Drive, Morgantown WV 26508, USA. Tel: 412-523-8284; Fax: 304-598-6442; E-mail: [email protected] Abstract mechanisms, prognosis, and management of these two conditions, especially in context of one another. Introduction: Two very interesting rheumatologic diagnoses include Systemic Lupus Erythematosus (SLE) and Anti-Neutrophil Case Report Cytoplasmic Antibody (ANCA)-negative Vasculitis. It is rare to see these two conditions diagnosed in the same patient. This case analyzes We present the case of a 69 year old female with two the complex interactions between these two disease states. simultaneous new diagnoses of SLE and ANCA-negative Vasculitis. Case: This is the case of a 69 year old female who presented with shortness of breath, hematuria, and renal failure. Lab work was pulmonaryHer past medical hypertension. history Herwas presentingsignificant forsymptoms hypothyroidism, included gastro esophageal reflux disease, and recently diagnosed antibody, normal complement levels and a negative Vasculitis panel. one week of increasing shortness of breath, pleuritic chest pain, Renalsignificant biopsy for was elevated consistent ANA (1:1280),with ANCA-associated positive double-stranded Vasculitis (AAV). -

Autoantibodies Diagnostic Tools for Autoimmune Disorders
Autoantibodies Diagnostic tools for autoimmune disorders What are autoantibodies? Despite these limitations, autoantibodies are a valuable tool for the diagnosis (when considered with other clinical Autoantibodies bind non-foreign structures within us and have and laboratory information) and monitoring of many been found in most well-defined autoimmune disorders. They autoimmune disorders. also occur in other disorders with an inflammatory component and even in some malignant disorders as paraneoplastic phenomena. How is tissue injury caused in With a few important exceptions, autoantibodies have no direct autoimmune disorders? Are role in pathogenesis and their main value is as a ‘marker’ adding autoantibodies always pathological? weight to a clinical diagnoses. Much tissue damage in autoimmune diseases is probably Circulating forms of autoantibodies may be detected by mediated by T cells and their effector mechanisms, rather than assays on serum. Tissue-bound antibodies may also be by B cells and their products, autoantibodies. Systemic lupus detected by direct immunofluorescence studies of non-fixed erythematosus and other connective tissue disorders are biopsy specimens. characterised by polyclonal self-reactive B cell expansions. Normal immune system functions include the recognition of, and Do autoantibodies ever occur naturally, discrimination between, self and non-self targets and unleashing without clinical associations? of effector mechanisms, such as complement proteins, cytotoxic T cells, cytokines and other phagocytic cells onto non-self Low-level autoantibodies occur naturally and more commonly in targets. persons who are older, female, have chronic diseases and often a family history of autoimmune abnormalities. These natural Autoantibody production is a consequence of ongoing autoantibodies occur in low concentrations and have weak recognition of self targets by both T and B cells. -

Colonic Ulcers in Propylthiouracil Induced Vasculitis with Secondary
338 ADVERSE DRUG REACTION Postgrad Med J: first published as 10.1136/pgmj.2004.026104 on 5 May 2005. Downloaded from Colonic ulcers in propylthiouracil induced vasculitis with secondary antiphospholipid syndrome P D Gaburri, J M F Chebli, Aˆ Attalla, C M N Pereira, H L Bonfante, E V Martins Junior, A K Gaburri ............................................................................................................................... Postgrad Med J 2005;81:338–340. doi: 10.1136/pgmj.2004.026104 migratory polyarthritis, and fever during the past 15 months. A 48 year old white woman was admitted to the hospital She was taking propylthiouracil 100 mg a day over a period because of several bouts of migratory polyarthritis, weight of three years for hyperthyroidism. The symptoms had loss, fever, and abdominal pain over a period of 15 months. worsened in the past two months and an intense deteriora- She had been taking propylthiouracil 100 mg daily for three tion of general physical state occurred. She needed frequent years for hyperthyroidism treatment. A test for antineutrophil use of opioids orally in the past two weeks to relieve cytoplasmic autoantibodies (ANCA) was positive with a abdominal pain. There was no blood or mucus in the stools. perinuclear pattern of staining. Antiphospholipid antibodies On admission she was pale—haemoglobin concentration were also detected. Colonoscopy showed several ulcers on 9.4 g%—and her temperature was 38˚C. Blood pressure was intestinal mucosa and the biopsy specimen showed intense 120/80 mm Hg and a painful hepatomegaly was noticed. The microscopic vasculitis. The patient is well after methylpredni- spleen was felt 7 cm below the left costal margin and an solone pulse therapy and eight months of oral azathioprine. -

Clinical and Laboratory Evaluation of Patients with Primary
original Article Clinical and laboratory evaluation of patients with primary antiphospholipid syndrome according to the frequency of antinuclear antibodies (ANA Hep-2) Jozélio Freire de Carvalho1,2, Maria Teresa Correia Caleiro2, Margarete Vendramini3, Eloísa Bonfá4 ABSTRACT Objective: To evaluate the frequency of clinical and laboratory manifestations in patients with primary antiphospholipid syndrome (PAPS) with positive antinuclear antibodies (ANA Hep-2+) compared to those in whom this antibody is ne- gative (ANA Hep-2-). Patients and Methods: This is a transversal study with 58 patients (82.8% females) with PAPS. Demographic and clinical data, comorbidities, medications, and antiphospholipid antibodies were evaluated. Results: Twenty (34.5%) out of 58 patients were positive for ANA Hep-2. Comparing the group of patients ANA Hep-2+ with those that were ANA Hep-2-, it was observed that both groups of patients with APS did not show statistically significant differences regarding demographic data, as well as the duration of the disease. As for clinical and laboratorial mani- festations, the ANA Hep-2+ group showed higher frequency of deep venous thrombosis (85 versus 52.6%, P = 0.04), a statistically higher frequency of anticardiolipin IgG (85 versus 52.6%, P = 0.02), and a tendency for anticardiolipin IgM (80% versus 52.6%, P = 0.05), as well as greater medians of those antibodies [33 (0-128) versus 20 (0-120) GPL, P = 0.008] and [33 (0-120) versus 18,5 (0-120) MPL, P = 0.009]. Such difference was not observed regarding other manifestations of APS, presence of comorbidities, lifestyle, and medications used. Conclusions: Patients with PAPS with ANA Hep-2+ have a higher frequency of deep venous thrombosis and anticardiolipin IgG and IgM.