(12) Patent Application Publication (10) Pub. No.: US 2015/0258127 A1 Terzi Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Expression of NOD2, NLRP3 and NLRC5 and Renal Injury in Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis
Wang et al. J Transl Med (2019) 17:197 https://doi.org/10.1186/s12967-019-1949-5 Journal of Translational Medicine RESEARCH Open Access The expression of NOD2, NLRP3 and NLRC5 and renal injury in anti-neutrophil cytoplasmic antibody-associated vasculitis Luo‑Yi Wang1,2,3, Xiao‑Jing Sun1,2,3, Min Chen1,2,3* and Ming‑Hui Zhao1,2,3,4 Abstract Background: Nucleotide‑binding oligomerization domain (NOD)‑like receptors (NLRs) are intracellular sensors of pathogens and molecules from damaged cells to regulate the infammatory response in the innate immune system. Emerging evidences suggested a potential role of NLRs in anti‑neutrophil cytoplasmic antibody (ANCA)‑associated vasculitis (AAV). This study aimed to investigate the expression of nucleotide‑binding oligomerization domain con‑ taining protein 2 (NOD2), NOD‑like receptor family pyrin domain containing 3 (NLRP3) and NOD‑like receptor family CARD domain containing 5 (NLRC5) in kidneys of AAV patients, and further explored their associations with clinical and pathological parameters. Methods: Thirty‑four AAV patients in active stage were recruited. Their renal specimens were processed with immu‑ nohistochemistry to assess the expression of three NLRs, and with double immunofuorescence to detect NLRs on intrinsic and infltrating cells. Analysis of gene expression was also adopted in cultured human podocytes. The associa‑ tions between expression of NLRs and clinicopathological parameters were analyzed. Results: The expression of NOD2, NLRP3 and NLRC5 was signifcantly higher in kidneys from AAV patients than those from normal controls, minimal change disease or class IV lupus nephritis. These NLRs co‑localized with podocytes and infltrating infammatory cells. -

Domino Effect in a Patient with Epstein-Barr Virus Infection and Autoimmunity: a Case Report Case Report Appointments Scheduled
International Journal of Case Report Clinical Rheumatology Domino effect in a patient with Epstein- Barr Virus infection and autoimmunity: A case report The link between autoimmune diseases and viral infections has been characterized, but specific Margarite Matossian, Carrie mechanisms behind this association remain a current area of investigation. Whether viral infections Crook, Alec Goldberg, Anna trigger or unmask autoimmunity, or if the pathologies occur concurrently, is not yet completely Stathopoulos, Justin Tien, understood. Specifically, Epstein - Barr virus (EBV) is implicated in several autoimmune disorders, Sheela Sheth, Osaid Saqqa & including Systemic Lupus Erythematosus (SLE). It is hypothesized that common immunologic Christopher Dale Shamburger* pathways are activated in the two pathologic states. This case report is an example of this confusing Department of Medicine, Tulane University presentation, and the importance of recognizing the association between autoimmunity and viral Health Sciences Center, New Orleans LA infections. This patient presented with symptoms concerning for SLE and hepatic autoimmunity 70115, USA with serology suggesting a recent infection with EBV. Given this complicated presentation, it is *Author for correspondence: difficult to determine which disease state presented first in patients with evidence of both SLE and EBV infection and whether this information is clinically relevant for ongoing treatment and [email protected] monitoring. Here, we provide an in-depth discussion of current genomic and immunological research that supports the associations amongst these disease pathologies. Introduction by an intricate psychiatric history. Then Autoimmune diseases have increased in we discuss the contribution of infection to frequency in industrialized countries over autoimmune disease states and important recent years [1]. The etiology of autoimmune distinctions between autoimmune diseases diseases, a self-reactive adaptive immune and autoinflammatory responses. -

Striking the Right Balance in Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis
Striking the Right Balance in Anti-neutrophil Cytoplasmic Antibody-Associated Vasculitis This symposium took place on 4th June 2021, as part of the European Alliance of Associations for Rheumatology (EULAR) virtual congress Speakers: Benjamin Terrier,¹ Joanna Robson,² Bernhard Hellmich³ 1. University of Paris and Hôpital Cochin, France 2. University of the West of England and Bristol Royal Infirmary, UK 3. University of Tübingen, Germany Disclosure: Terrier has been an advisory board member and/or received consulting fees/ travel expenses from AstraZeneca, Chugai, Grifols, GlaxoSmithKline, Janssen, LFB, Octapharma, Roche, and Vifor Pharma. Robson has received speaker’s fees from Roche and Vifor Pharma; and research support from Vifor Pharma. Hellmich has been an investigator in clinical trials for Ab2Bio, AbbVie, AstraZeneca, Bristol- Myers Squibb, Chemocentrix, GlaxoSmithKline, InflaRx, Kiniksa, Nippon Kayaku, Novartis, Roche, and Sanofi. He has acted as a consultant, advisory board member, and/or lecturer for AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Chugai, GlaxoSmithKline, InflaRx, Novartis, Pfizer, Roche, and Vifor Pharma. He is also a member of the Guideline Committees for European Alliance of Associations for Rheumatology (EULAR) and the German Society of Rheumatology (DGRh). Acknowledgements: Writing assistance was provided by Helen Boreham. Support: The publication of this article was funded by Vifor Pharma. The views and opinions expressed are those of the presenters. Content was reviewed by Vifor Pharma for medical accuracy. Citation: Rheumatol. 2021;8[1]:43-50. Meeting Summary Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) causes irreversible short- and long-term damage to vital organs, particularly the kidneys and lungs. Current standard of care (SOC) for AAV, of which glucocorticoids (GC) are a lynchpin, has a number of important limitations: responses to therapy are variable, some patients fail to achieve and sustain remission, and treatment related adverse events (AE) are common. -

Pediatric Motor Inflammatory Neuropathy: the Role Of
brain sciences Case Report Pediatric Motor Inflammatory Neuropathy: The Role of Antiphospholipid Antibodies Claudia Brogna 1,2,*, Marco Luigetti 3 , Giulia Norcia 1, Roberta Scalise 1, Gloria Ferrantini 1, Beatrice Berti 1, Domenico M. Romeo 4, Raffaele Manna 5, Eugenio Mercuri 1 and Marika Pane 1 1 Pediatric Neurology and Nemo Clinical Centre, Fondazione Policlinico Universitario A. Gemelli IRCSS, Università Cattolica del Sacro Cuore, 00168 Roma, Italy; [email protected] (G.N.); [email protected] (R.S.); [email protected] (G.F.); [email protected] (B.B.); [email protected] (E.M.); [email protected] (M.P.) 2 Pediatric Neuropsichiatric Unit, ASL, 83100 Avellino, Italy 3 Institute of Neurology, Fondazione Policlinico Universitario A. Gemelli IRCSS, Università Cattolica del sacro Cuore, 00168 Roma, Italy; [email protected] 4 Pediatric Neurology, Fondazione Policlinico Universitario A. Gemelli IRCSS, Università Cattolica del Sacro Cuore, 00168 Roma, Italy; [email protected] 5 Periodic Fevers Research Centre and rare disease. Department of Internal Medicine, Fondazione Policlinico Universitario A. Gemelli IRCSS, Università Cattolica del Sacro Cuore, 00168 Roma, Italy; raff[email protected] * Correspondence: [email protected] Received: 4 February 2020; Accepted: 5 March 2020; Published: 7 March 2020 Abstract: We report the clinical case of a nine-year-old girl who presented with progressive motor neuropathy, revealed via the detection of a higher delay in F-wave recording using digitalized nerve conduction/electromyography. Since the lupus anticoagulant (LAC) positivity, detected using diluted Russell viper venom time (dRVVT), switched to persistent serological anticardiolipin immunoglobulin G (IgG) positivity, a possible non-thrombotic antiphospholipid antibody (aPL)-related clinical manifestation was suspected, and intravenous immunoglobulin treatment (IVIG) was started. -
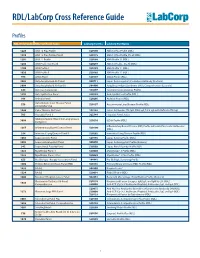
RDL/Labcorp Cross Reference Guide
RDL/LabCorp Cross Reference Guide Profiles RDL Order Code RDL Test/Panel Name LabCorp Test No. LabCorp Test Name 1228 ANA 12 Plus Profile 520180 ANA 12 Plus Profile (RDL) 1230 ANA 12 Plus Profile, Do All 520175 ANA 12 Plus Profile, Do all (RDL) 1201 ANA 12 Profile 520188 ANA Profile 12 (RDL) 1206 ANA Profile 12, Do All 520299 ANA 12 Profile , Do All (RDL) 1100 ANA Profile I 520185 ANA Profile 11 (RDL) 1020 ANA Profile II 520185 ANA Profile 11 (RDL) 994 ANCA Panel 520149 ANCA Profile (RDL) 3003 Antiphospholipid Ab Panel I 500711 Lupus Anticoagulant/Cardiolipin Antibody (Esoterix) 3004 Antiphospholipid Ab Panel II 504400 Antiphospholipid Syndrome (APS), Comprehensive (Esoterix) 644 Anti-Saccharomyces 164657 Saccharomyces cerevisiae Profile 1292 Anti-Synthetase Panel 520193 Anti-Synthetase Profile (RDL) 996 Arthritis Panel 520205 Arthritis Profile (RDL) Autoimmune Liver Disease Panel, 558 520197 Autoimmune Liver Disease Profile (RDL) Comprehensive 1044 Celiac Disease Ab Panel 165142 Celiac Antibodies tTG IgA, EMA IgA, Total IgA with Reflex to tTG IgG 705 Hepatitis Panel II 322744 Hepatitis Panel, Acute ILDdxComplete (Interstitial Lung Disease 3008 520210 ILDdx Profile (RDL) Complete) Inflammatory Bowel Disease (IBD) Profile with Anti-Pancreatic Antibodies 1265 Inflammatory Bowel Disease Panel 520190 (RDL) 354 Interstitial Lung Disease Panel II 520202 Interstitial Lung Disease Profile (RDL) 1065 Lupus Activity Panel 520195 Lupus Activity Profile (RDL) 3002 Lupus Anticoagulant Panel 500070 Lupus Anticoagulant Profile (Esoterix) 342 Lupus Renal -
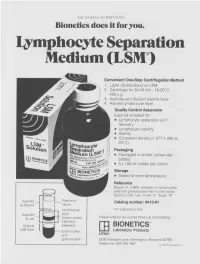
Lymphocyte Separation Medium (LSM
THE JOURNAL OF IMMUNOLOGY Bionetics does it for you. Lymphocyte Separation Medium (LSM wenient One-Step Centrifugation Method _ayer diluted blood on LSM. 2,entrifuge for 30-40 min., 18-20°C, ~.00 x g. ~,spirate and discard plasma layer -larvest lymphocyte layer. Quality Control Assurance Each lot is tested for: • Lymphocyte separation and recovery. • Lymphocyte viability. • Sterility. • Consistent density (1.077-1.080 at 20°C). Packaging • Packaged in amber, screw-cap bottles. • 5 x 100 ml bottles per carton. Storage. • Stored at room temperature. Reference Boyum, A. (1968): Isolation of mononuclear cells and granulocytes from human blood. Scand J. Clin. Lab. Invest. 21, Suppl. 97. Aspirate I IC[OI I IC~ LJI Catalog number: 8410-01 & discard serum Lymphocyte For Laboratory Use Aspirate layer & use (mononuclear Please write for our current Price List and Catalog. cells and Original platelets) ITi BIONETICS° LSM layer Erythrocytes Laboratory Products and Litton granulocytes 5516 Nicholson Lane, Kensington, Maryland 20795 Telephone: (301) 881-1557 1979 Litton Bionetics, tnc Get the most out of your high quality cytotoxic antibodies with LOW-TOX-M RABBIT COMPLEMENT LOW TOXICITY HIGH ACTIVITY Presentation: CL 3051 5 x 1 ml, lyophilized $30.00 When it comes to COMPLEMENT... come to CEDARLANE Direct orders or inquiries to: UNITED STATES: WORLDWIDE EXCEPT U.S. ,4 C~L CEDARLANE ACCURATE CHEMICAL & LABORATO RI ES SCIENTIFIC CORPORATION LIMITED 5516-8TH LINE, R.R. 2 28 TEC STREET, HICKSVtLLE, N.Y. 11801 HORNBY, ONTARIO, CANADA LOP 1E0 Telephone -

32 Mechanism of Thrombosis in Antiphospholipid Syndrome: Binding to Platelets Joan-Carles Reverter and Dolors Tàssies
32 Mechanism of Thrombosis in Antiphospholipid Syndrome: Binding to Platelets Joan-Carles Reverter and Dolors Tàssies Introduction Antiphospholipid antibodies (aPL) are related to thrombosis in the antiphospho- lipid syndrome (APS) [1, 2] and numerous pathophysiological mechanisms have been suggested involving cellular effects, plasma coagulation regulatory proteins, and fibrinolysis [3, 4]: aPL may act as blocking agents directly inhibiting antigen enzymatic or co-factor function of hemostasis; may bind fluid-phase antigens of hemostasis involved proteins and then decrease plasma antigen levels by clearance of immune complexes; may form immune complexes with their antigens that may be deposited in blood vessels causing inflammation and tissue injury; may cause dysregulation of antigen–phospholipid binding due to cross-linking of membrane bound antigens; and may trigger cell mediated events by cross-linking of antigen bound to cell surfaces or cell surface receptors [3, 4]. Moreover, several characteris- tics of the aPL, such as the concentration, class/subclass, affinity or charge, and several characteristics of the antigens, as the concentration, size, location or charge, may influence which of the theoretical autoantibody actions will occur in vivo [3]. Among the cellular mechanisms supposed to be involved, platelets have been considered as one of the most promising potential target for circulating aPL that may cause antibody mediated thrombosis as a part of the clinical spectrum of the autoimmune disorder of the APS. In the present chapter we will focus on the inter- actions that involve aPL binding to platelet membrane or platelet membrane bound antigens. Platelets as Target for aPL Platelets play a central role in primary hemostasis involving platelet adhesion to the injured blood vessel wall, followed by platelet activation, granule release, shape change, and rearrangement of the outer membrane phospholipids and proteins, transforming them into a highly efficient procoagulant surface [5]. -

Induction of Protein Citrullination and Auto-Antibodies Production In
www.nature.com/scientificreports OPEN Induction of protein citrullination and auto-antibodies production in murine exposed to nickel Received: 11 November 2015 Accepted: 21 December 2017 nanomaterials Published: xx xx xxxx Bashir M. Mohamed1,7, Noreen T. Boyle2,3, Anja Schinwald4, Bruno Murer5, Ronan Ward6, Omar K. Mahfoud1, Tatsiana Rakovich1, Kieran Crosbie-Staunton1, Steven G. Gray 7, Ken Donaldson4, Yuri Volkov1,2 & Adriele Prina-Mello 1,2 Citrullination, or the post-translational deimination of polypeptide-bound arginine, is involved in several pathological processes in the body, including autoimmunity and tumorigenesis. Recent studies have shown that nanomaterials can trigger protein citrullination, which might constitute a common pathogenic link to disease development. Here we demonstrated auto-antibody production in serum of nanomaterials-treated mice. Citrullination-associated phenomena and PAD levels were found to be elevated in nanomaterials -treated cell lines as well as in the spleen, kidneys and lymph nodes of mice, suggesting a systemic response to nanomaterials injection, and validated in human pleural and pericardial malignant mesothelioma (MM) samples. The observed systemic responses in mice exposed to nanomaterials support the evidence linking exposure to environmental factors with the development of autoimmunity responses and reinforces the need for comprehensive safety screening of nanomaterials. Furthermore, these nanomaterials induce pathological processes that mimic those observed in Pleural MM, and therefore require further investigations into their carcinogenicity. Citrullination is involved in several pathological processes in the body, including autoimmunity and tumor- igenesis. Citrullinated proteins are generated by a post-translational deimination or demethylimination of polypeptide-bound arginine by a family of Ca2+-dependent enzyme peptidylarginine deiminase (PAD)1. -

Heritability of Autoantibody Levels in a Twin Population
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2009 Heritability of Autoantibody Levels in a Twin Population Amal Rastogi Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Periodontics and Periodontology Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/1854 This Thesis is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. School of Dentistry Virginia Commonwealth University This is to certify that the thesis prepared by Amal Rastogi, DMD, PhD entitled HERITABILITY OF AUTOANTIBODY LEVELS IN A TWIN POPULATION has been approved by his committee as satisfactory completion of the thesis requirement for the degree of Master of Science in Dentistry. John Gunsolley, DDS, MS, Professor, Department of Periodontics, VCU, School of Dentistry Harvey Schenkein, DDS, PhD, Chair, Department of Periodontics, VCU, School of Dentistry Robert Sabatini, DDS, MS, Assistant Professor, Department of Periodontics, VCU, School of Dentistry Thomas Waldrop, DDS, MS, Graduate Director, Department of Periodontics, VCU, School of Dentistry Harvey Schenkein, DDS, PhD, Chair, Department of Periodontics, VCU, School of Dentistry Laurie Carter, DDS, PhD, Director of Advanced Dental Education,VCU, School of Dentistry Dr. F. Douglas Boudinot, Dean of the Graduate School, VCU June 29, 2009 © Amal Rastogi, DMD, PhD 2009 All Rights Reserved 2 HERITABILITY OF AUTOANTIBODY LEVELS IN A TWIN POPULATION A thesis submitted in partial fulfillment of the requirements for the degree of MSD at Virginia Commonwealth University. -

Antibody (ANCA)-Associated Vasculitis Avacopan Introduction
CO-1 Avacopan for the Treatment of Anti-Neutrophil Cytoplasmic Auto- antibody (ANCA)-Associated Vasculitis ChemoCentryx, Inc. Arthritis Advisory Committee May 6, 2021 CO-2 Avacopan Introduction Thomas J. Schall, Ph.D. President, Chief Executive Officer ChemoCentryx, Inc. CO-3 Avacopan: First-in-Class, Targeted Therapy for ANCA-Associated Vasculitis ANCA-associated vasculitis is rare, severe, and often fatal autoimmune disease Anti-neutrophil cytoplasmic auto-antibodies (ANCA) involved in pathogenesis Inflammation of small vessels, can affect any organ Commonly affects kidneys Glucocorticoid treatment associated with significant toxicities Despite current therapies, > 1 in 10 patients die within first year of diagnosis1,2 1. Heijl et al., 2017; 2. Little et al., 2010 CO-4 Central Role of C5a in Pathogenesis of ANCA-Associated Vasculitis Jennette and Falk, 2014 CO-5 Avacopan: Highly Potent and Selective C5aR Inhibitor Avacopan avoids long-term 1 biological consequences of ‘upstream’ complement inhibition 1 Does not block C5b-9 production; leaves host defense mechanism C5aC5a AntibodiesAntibodies 2 membrane attack complex (MAC) in place C6-C9C6-C9 AvacopanAvacopan Preserves beneficial 3 functions of C5L2 pathway C5aR Leukocyte migration and signaling Leukocyte trafficking, Cell lysis migration and (i.e., Neisseria control activation Beneficial Targets ‘downstream' anti-inflammatoryanti-inflammatory 4 effect complement pathway Adaptive Phagocytosis 4 3 2 signaling and clearance CO-6 Avacopan in ANCA-Associated Vasculitis Pirow Bekker, MD, PhD Clinical Lead Avacopan Clinical Development Program ChemoCentryx, Inc. CO-7 Avacopan Proposed Indication and Dose Proposed Indication …for the treatment of adult patients with anti-neutrophil cytoplasmic auto-antibody (ANCA)-associated vasculitis (granulomatosis with polyangiitis and microscopic polyangiitis). -

Degradation of Neutrophil Extracellular Traps Is Decreased in Patients with Antiphospholipid Syndrome J
Degradation of neutrophil extracellular traps is decreased in patients with antiphospholipid syndrome J. Leffler1, L. Stojanovich2, Y. Shoenfeld3, G. Bogdanovic2, R. Hesselstrand4, A.M. Blom1 1Dept. of Laboratory Medicine, Section of Medical Protein Chemistry, Lund University, Malmö, Sweden; 2Dept. of Internal Medicine, “Bezhanijska Kosa” University Medical Center, Belgrade, Serbia; 3Zabludowicz Center for Autoimmune Diseases, Sheba Medical Center, Tel Aviv, Israel; 4Dept. of Clinical Sciences, Section of Rheumatology, Lund University, Lund, Sweden. Abstract Objective A decreased ability to degrade neutrophil extracellular traps (NETs) is seen in a subgroup of patients with systemic lupus erythematosus (SLE) and correlates with the presence of autoantibodies. Antiphospholipid syndrome (APS) can develop secondary to SLE or as a primary disease. In the current study we investigated the ability of sera from patients with APS to degrade NETs. The presence of antibodies against NETs and neutrophil remnants were also determined in the same patients. Methods In the study, 106 patients with APS (73 primary and 33 secondary), 76 patients with systemic sclerosis (SSc) and 77 healthy donors as control samples were included. NETs generated from neutrophils isolated from healthy volunteers were incubated with patient sera, followed by measurement of degraded NETs or deposited IgG. Results Sera of APS patients had a decreased ability to degrade NETs compared to healthy controls, with no difference between primary and secondary APS. Sera from SSc patients did not differ significantly from healthy controls in the ability to degrade NETs. A decreased degradation of NETs correlated weakly to increased levels of antibodies against NETs/ neutrophil remnants in patients with primary APS, but stronger in patients with secondary APS. -

Belimumab (Benlysta®)
Policy Medical Policy Manual Approved Revision: Do Not Implement until 8/31/21 Belimumab (Benlysta®) NDC CODE(S) 49401-0101-XX BENLYSTA 120MG Solution Reconstituted (GLAXO SMITH KLINE) 49401-0102-XX BENLYSTA 400MG Solution Reconstituted (GLAXO SMITH KLINE) DESCRIPTION Belimumab is a human IgG1 monoclonal antibody specific for soluble human B lymphocyte stimulator protein (BLyS), a B cell survival factor. It is produced by recombinant DNA technology in a mammalian cell expression system. Belimumab does not bind to B cells directly but blocks access of soluble BLyS to its receptors on B cells. This inhibits the survival of B cells and reduces the differentiation of B cells into immunoglobulin-producing plasma cells. Treatment with belimumab leads to reductions in circulating CD19+, CD20+, naïve and activated B cells along with plasmacytoid cells and the systemic lupus erythematosus (SLE) B-cell subset. POLICY Belimumab for the treatment of the following is considered medically necessary if the medical appropriateness criteria are met. (See Medical Appropriateness below.) o Systemic Lupus Erythematosus (SLE) o Lupus Nephritis Belimumab or the treatment of other conditions/diseases, including, but not limited to, Active Central Nervous System Lupus is considered investigational. MEDICAL APPROPRIATENESS INITIAL APPROVAL CRITERIA Patient is at least 18 years of age (unless otherwise specified); AND Universal Criteria Patient must not have an active infection; AND Patient has not received a live vaccine within 30 days before starting or