32 Mechanism of Thrombosis in Antiphospholipid Syndrome: Binding to Platelets Joan-Carles Reverter and Dolors Tàssies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Disseminated Intravascular Coagulation (DIC) and Thrombosis: the Critical Role of the Lab Paul Riley, Phd, MBA, Diagnostica Stago, Inc
Generate Knowledge Disseminated Intravascular Coagulation (DIC) and Thrombosis: The Critical Role of the Lab Paul Riley, PhD, MBA, Diagnostica Stago, Inc. Learning Objectives Describe the basic pathophysiology of DIC Demonstrate a diagnostic and management approach for DIC Compare markers of thrombin & plasmin generation in DIC, including D-Dimer, fibrin monomers (FM; aka soluble fibrin monomers, SFM), and fibrin degradation products (FDPs; aka fibrin split products, FSPs) Correlate DIC theory and testing to specific clinical cases DIC = Death is Coming What is Hemostasis? Blood Circulation Occurs through blood vessels ARTERIES The heart pumps the blood Arteries carry oxygenated blood away from the heart under high pressure VEINS Veins carry de-oxygenated blood back to the heart under low pressure Hemostasis The mechanism that maintains blood fluidity Keeps a balance between bleeding and clotting 2 major roles Stop bleeding by repairing holes in blood vessels Clean up the inside of blood vessels Removes temporary clot that stopped bleeding Sweeps off needless deposits that may cause blood flow blockages Two Major Diseases Linked to Hemostatic Abnormalities Bleeding = Hemorrhage Blood clot = Thrombosis Physiology of Hemostasis Wound Sealing break in vesselEFFRAC PRIMARY PLASMATIC HEMOSTASIS COAGULATION strong clot wound sealing blood FIBRINOLYSIS flow ± stopped clot destruction The Three Steps of Hemostasis Primary Hemostasis Interaction between vessel wall, platelets and adhesive proteins platelet clot Coagulation Consolidation -

Domino Effect in a Patient with Epstein-Barr Virus Infection and Autoimmunity: a Case Report Case Report Appointments Scheduled
International Journal of Case Report Clinical Rheumatology Domino effect in a patient with Epstein- Barr Virus infection and autoimmunity: A case report The link between autoimmune diseases and viral infections has been characterized, but specific Margarite Matossian, Carrie mechanisms behind this association remain a current area of investigation. Whether viral infections Crook, Alec Goldberg, Anna trigger or unmask autoimmunity, or if the pathologies occur concurrently, is not yet completely Stathopoulos, Justin Tien, understood. Specifically, Epstein - Barr virus (EBV) is implicated in several autoimmune disorders, Sheela Sheth, Osaid Saqqa & including Systemic Lupus Erythematosus (SLE). It is hypothesized that common immunologic Christopher Dale Shamburger* pathways are activated in the two pathologic states. This case report is an example of this confusing Department of Medicine, Tulane University presentation, and the importance of recognizing the association between autoimmunity and viral Health Sciences Center, New Orleans LA infections. This patient presented with symptoms concerning for SLE and hepatic autoimmunity 70115, USA with serology suggesting a recent infection with EBV. Given this complicated presentation, it is *Author for correspondence: difficult to determine which disease state presented first in patients with evidence of both SLE and EBV infection and whether this information is clinically relevant for ongoing treatment and [email protected] monitoring. Here, we provide an in-depth discussion of current genomic and immunological research that supports the associations amongst these disease pathologies. Introduction by an intricate psychiatric history. Then Autoimmune diseases have increased in we discuss the contribution of infection to frequency in industrialized countries over autoimmune disease states and important recent years [1]. The etiology of autoimmune distinctions between autoimmune diseases diseases, a self-reactive adaptive immune and autoinflammatory responses. -

Pediatric Motor Inflammatory Neuropathy: the Role Of
brain sciences Case Report Pediatric Motor Inflammatory Neuropathy: The Role of Antiphospholipid Antibodies Claudia Brogna 1,2,*, Marco Luigetti 3 , Giulia Norcia 1, Roberta Scalise 1, Gloria Ferrantini 1, Beatrice Berti 1, Domenico M. Romeo 4, Raffaele Manna 5, Eugenio Mercuri 1 and Marika Pane 1 1 Pediatric Neurology and Nemo Clinical Centre, Fondazione Policlinico Universitario A. Gemelli IRCSS, Università Cattolica del Sacro Cuore, 00168 Roma, Italy; [email protected] (G.N.); [email protected] (R.S.); [email protected] (G.F.); [email protected] (B.B.); [email protected] (E.M.); [email protected] (M.P.) 2 Pediatric Neuropsichiatric Unit, ASL, 83100 Avellino, Italy 3 Institute of Neurology, Fondazione Policlinico Universitario A. Gemelli IRCSS, Università Cattolica del sacro Cuore, 00168 Roma, Italy; [email protected] 4 Pediatric Neurology, Fondazione Policlinico Universitario A. Gemelli IRCSS, Università Cattolica del Sacro Cuore, 00168 Roma, Italy; [email protected] 5 Periodic Fevers Research Centre and rare disease. Department of Internal Medicine, Fondazione Policlinico Universitario A. Gemelli IRCSS, Università Cattolica del Sacro Cuore, 00168 Roma, Italy; raff[email protected] * Correspondence: [email protected] Received: 4 February 2020; Accepted: 5 March 2020; Published: 7 March 2020 Abstract: We report the clinical case of a nine-year-old girl who presented with progressive motor neuropathy, revealed via the detection of a higher delay in F-wave recording using digitalized nerve conduction/electromyography. Since the lupus anticoagulant (LAC) positivity, detected using diluted Russell viper venom time (dRVVT), switched to persistent serological anticardiolipin immunoglobulin G (IgG) positivity, a possible non-thrombotic antiphospholipid antibody (aPL)-related clinical manifestation was suspected, and intravenous immunoglobulin treatment (IVIG) was started. -
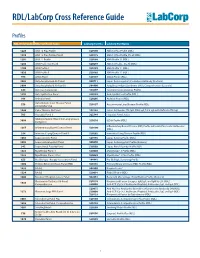
RDL/Labcorp Cross Reference Guide
RDL/LabCorp Cross Reference Guide Profiles RDL Order Code RDL Test/Panel Name LabCorp Test No. LabCorp Test Name 1228 ANA 12 Plus Profile 520180 ANA 12 Plus Profile (RDL) 1230 ANA 12 Plus Profile, Do All 520175 ANA 12 Plus Profile, Do all (RDL) 1201 ANA 12 Profile 520188 ANA Profile 12 (RDL) 1206 ANA Profile 12, Do All 520299 ANA 12 Profile , Do All (RDL) 1100 ANA Profile I 520185 ANA Profile 11 (RDL) 1020 ANA Profile II 520185 ANA Profile 11 (RDL) 994 ANCA Panel 520149 ANCA Profile (RDL) 3003 Antiphospholipid Ab Panel I 500711 Lupus Anticoagulant/Cardiolipin Antibody (Esoterix) 3004 Antiphospholipid Ab Panel II 504400 Antiphospholipid Syndrome (APS), Comprehensive (Esoterix) 644 Anti-Saccharomyces 164657 Saccharomyces cerevisiae Profile 1292 Anti-Synthetase Panel 520193 Anti-Synthetase Profile (RDL) 996 Arthritis Panel 520205 Arthritis Profile (RDL) Autoimmune Liver Disease Panel, 558 520197 Autoimmune Liver Disease Profile (RDL) Comprehensive 1044 Celiac Disease Ab Panel 165142 Celiac Antibodies tTG IgA, EMA IgA, Total IgA with Reflex to tTG IgG 705 Hepatitis Panel II 322744 Hepatitis Panel, Acute ILDdxComplete (Interstitial Lung Disease 3008 520210 ILDdx Profile (RDL) Complete) Inflammatory Bowel Disease (IBD) Profile with Anti-Pancreatic Antibodies 1265 Inflammatory Bowel Disease Panel 520190 (RDL) 354 Interstitial Lung Disease Panel II 520202 Interstitial Lung Disease Profile (RDL) 1065 Lupus Activity Panel 520195 Lupus Activity Profile (RDL) 3002 Lupus Anticoagulant Panel 500070 Lupus Anticoagulant Profile (Esoterix) 342 Lupus Renal -

Heart – Thrombus
Heart – Thrombus Figure Legend: Figure 1 Heart, Atrium - Thrombus in a male Swiss Webster mouse from a chronic study. A thrombus is present in the right atrium (arrow). Figure 2 Heart, Atrium - Thrombus in a male Swiss Webster mouse from a chronic study (higher magnification of Figure 1). Multiple layers of fibrin, erythrocytes, and scattered inflammatory cells (arrows) comprise this right atrial thrombus. Figure 3 Heart, Atrium - Thrombus in a male Swiss Webster mouse from a chronic study. A large thrombus with two foci of mineral fills the left atrium (arrow). Figure 4 Heart, Atrium - Thrombus in a male Swiss Webster mouse from a chronic study (higher magnification of Figure 3). This thrombus in the left atrium (arrows) has two dark, basophilic areas of mineral (arrowheads). 1 Heart – Thrombus Comment: Although thrombi can be seen in the right (Figure 1 and Figure 2) or left (Figure 3 and Figure 4) atrium, the most common site of spontaneously occurring and chemically induced thrombi is the left atrium. In acute situations, the lumen is distended by a mass of laminated fibrin layers, leukocytes, and red blood cells. In more chronic cases, there is more organization of the thrombus (e.g., presence of vascularized fibrous connective tissue, inflammation, and/or cartilage metaplasia), with potential attachment to the atrial wall. Spontaneous rates of cardiac thrombi were determined for control Fischer 344 rats and B6C3F1 mice: in 90-day studies, 0% in rats and mice; in 2-year studies, 0.7% in both genders of mice, 4% in male rats, and 1% in female rats. -

Thrombotic Thrombocytopenic Purpura
Thrombotic thrombocytopenic Purpura Flora Peyvandi Angelo Bianchi Bonomi Hemophilia and Thrombosis Center IRCCS Ca’ Granda Ospedale Maggiore Policlinico University of Milan Milan, Italy Disclosures Research Support/P.I. No relevant conflicts of interest to declare Employee No relevant conflicts of interest to declare Consultant Kedrion, Octapharma Major Stockholder No relevant conflicts of interest to declare Speakers Bureau Shire, Alnylam Honoraria No relevant conflicts of interest to declare Scientific Advisory Ablynx, Shire, Roche Board Objectives • Advances in understanding the pathogenetic mechanisms and the resulting clinical implications in TTP • Which tests need to be done for diagnosis of congenital and acquired TTP • Standard and novel therapies for congenital and acquired TTP • Potential predictive markers of relapse and implications on patient management during remission Thrombotic Thrombocytopenic Purpura (TTP) First described in 1924 by Moschcowitz, TTP is a thrombotic microangiopathy characterized by: • Disseminated formation of platelet- rich thrombi in the microvasculature → Tissue ischemia with neurological, myocardial, renal signs & symptoms • Platelets consumption → Severe thrombocytopenia • Red blood cell fragmentation → Hemolytic anemia TTP epidemiology • Acute onset • Rare: 5-11 cases / million people / year • Two forms: congenital (<5%), acquired (>95%) • M:F ratio 1:3 • Peak of incidence: III-IV decades • Mortality reduced from 90% to 10-20% with appropriate therapy • Risk of recurrence: 30-35% Peyvandi et al, Haematologica 2010 TTP clinical features Bleeding 33 patients with ≥ 3 acute episodes + Thrombosis “Old” diagnostic pentad: • Microangiopathic hemolytic anemia • Thrombocytopenia • Fluctuating neurologic signs • Fever • Renal impairment ScullyLotta et et al, al, BJH BJH 20122010 TTP pathophysiology • Caused by ADAMTS13 deficiency (A Disintegrin And Metalloproteinase with ThromboSpondin type 1 motifs, member 13) • ADAMTS13 cleaves the VWF subunit at the Tyr1605–Met1606 peptide bond in the A2 domain Furlan M, et al. -

Thrombosis and Embolism After Injury J Clin Pathol: First Published As 10.1136/Jcp.S3-4.1.86 on 1 January 1970
J. clin. Path., 23, Suppl. (Roy. Coll. Path.), 4, 86-101 Thrombosis and embolism after injury J Clin Pathol: first published as 10.1136/jcp.s3-4.1.86 on 1 January 1970. Downloaded from S. SEVITT From the Birmingham Accident Hospital Thrombosis is frequent in injured patients. It classified as follows, namely, local thrombosis, takes different forms, and at least one of them, deep vein thrombosis, pulmonary microem- deep vein thrombosis in the lower limbs, is a bolism, glomerular microthrombosis, allied to common cause of morbidity and death through the Schwartzman reaction, occasional cases of embolic detachment. The different kinds may be arterial thrombosis, and rarely, abacterial vege- tative endocarditis. Thrombi form in flowing blood and are layered structures, unlike blood clots which form copyright. in static blood. They contain platelets, fibrin, red cells, and leucocytes, or a variable mixture, the differences depending on size, genesis, age, and venous or arterial location; but whatever the origin, the building blocks of enlarging thrombi are closely packed clumps of platelets with narrow fibrin borders (Fig. 1). Two main pro- http://jcp.bmj.com/ cesses are involved, namely, coagulation and platelet aggregation. These are interlinked and local release of thrombin is probably the key factor; thrombin promotes platelet clumping at a low concentration and fibrin formation at a higher concentration. Further, the release of substances from platelets can set in motion the coagulation on September 30, 2021 by guest. Protected process. Local Thrombosis and Haemostasis Thrombosis is frequent as a direct response to injury. In burned skin, for example, small venous thrombi may become prominent in the subdermis and subcutaneous tissue. -

Significant Factors Associated with Lupus Nephritis P Alba, L Bento, M J Cuadrado, Y Karim, M F Tungekar, I Abbs, M a Khamashta, D D’Cruz,Grvhughes
556 EXTENDED REPORT Ann Rheum Dis: first published as 10.1136/ard.62.6.556 on 1 June 2003. Downloaded from Anti-dsDNA, anti-Sm antibodies, and the lupus anticoagulant: significant factors associated with lupus nephritis P Alba, L Bento, M J Cuadrado, Y Karim, M F Tungekar, I Abbs, M A Khamashta, D D’Cruz,GRVHughes ............................................................................................................................. Ann Rheum Dis 2003;62:556–560 Background: Lupus nephritis (LN) is a common manifestation in patients with systemic lupus erythema- tosus (SLE). Autoantibodies and ethnicity have been associated with LN, but the results are controver- sial. Objective: To study the immunological and demographic factors associated with the development of LN. Patients and methods: A retrospective case-control study of 127 patients with biopsy-proven LN, and 206 randomly selected patients with SLE without nephritis as controls was designed. All patients had attended our lupus unit during the past 12 years. Standard methods were used for laboratory testing. Results: Patients with LN were significantly younger than the controls at the time of SLE diagnosis See end of article for (mean (SD) 25.6 (8.8) years v 33.7 (12.5) years; p<0.0001). The proportion of patients of black eth- authors’ affiliations nic origin was significantly higher in the group with nephritis (p=0.02). There were no differences in ....................... sex distribution or duration of follow up. A higher proportion of anti-dsDNA, anti-RNP, anti-Sm, and Correspondence to: lupus anticoagulant (LA) was seen in the group with nephritis (p=0.002; p=0.005; p=0.0001; Dr M J Cuadrado, Lupus p=0.01, respectively). -

Hepatic Manifestations of the Antiphospholipid Syndrome
EDITORIAL Hepatic Manifestations of the Antiphospholipid Syndrome Key words: autoimmune hepatitis, Sjogren's syndrome, Budd- autoimmune hepatitis (7). Examinations prior to liver biopsy Chiari syndrome, nodular regenerative hyperpla- revealed a greatly prolonged APTT,and the patient was found sia to be positive for IgG anticardiolipin antibody and lupus anti- coagulant. However, this patient showedno signs of thrombo- sis or any other symptomssuggestive ofAPS. In this issue, Katayama et al (8) report a case of Sjogren's Antiphospholipid syndrome (APS) is characterized by the syndrome complicated with autoimmune hepatitis and APS. presence of antiphospholipid antibodies (anticardiolipin anti- bodies and/or lupus anticoagulant) in association with symp- See also p 73. toms including venous/arterial thromboses, recurrent fetal loss and thrombocytopenia. Thromboticepisodes mayoccur at any In previously reported cases of autoimmune hepatitis asso- part of the circulation system, hence, any organ may be in- ciated with the presence of antiphospholipid antibodies, anti- volved. As for hepatic involvement, the most well knownmani- nuclear antibody was present, but the presence of anti-SS-A festation ofAPSis Budd-Chiari syndrome, which is caused antibody or sicca symptomshave not been documented. There- mostly by obstruction of hepatic veins or inferior vena cava. fore, this case may possibly be the first documented case of Pelletier et al (1) have reported that of their 22 non-tumourous autoimmune hepatitis with APSand Sjogren's syndrome. Their Budd-Chiari patients, 4 had antiphospholipid antibodies with- patient had autoimmune hepatitis, and Sjogren's syndrome out other causes of hepatic vein thrombosis. Amongthese 4 confirmed by Schirmer's test and sialography. The patient patients, 2 seemed to have primary APS, i.e., no signs of other showed positive for (32-GPI dependent anticardiolipin antibody autoimmunediseases such as SLE. -

Zebrafish Depends on Von Willebrand Factor
Hemostasis ARTICLE Histone-induced thrombotic thrombocytopenic Ferrata Storti Foundation purpura in adamts13-/- zebrafish depends on von Willebrand factor Liang Zheng,1 Mohammad S. Abdelgawwad,1 Di Zhang,1 Leimeng Xu,1 Shi Wei,2 Wenjing Cao1 and X. Long Zheng1 Divisions of 1Laboratory Medicine and 2Anatomic Pathology, Department of Pathology, The University of Alabama at Birmingham, Birmingham, AL, USA ABSTRACT Haematologica 2020 Volume 105(4):1107-1119 hrombotic thrombocytopenic purpura (TTP) is caused by severe deficiency of ADAMTS13 (A13), a plasma metalloprotease that Tcleaves endothelium-derived von Willebrand factor (VWF). However, severe A13 deficiency alone is often not sufficient to cause an acute TTP; additional factors may be required to trigger the disease. Using CRISPR/Cas9, we created and characterized several novel zebrafish lines carrying a null mutation in a13-/-, vwf, and both. We fur- ther used these zebrafish lines to test the hypothesis that inflammation that results in neutrophil activation and release of histone/DNA com- plexes may trigger TTP. As shown, a13-/- zebrafish exhibit increased lev- els of plasma VWF antigen, multimer size, and ability of thrombocytes to adhere to a fibrillar collagen-coated surface under flow. The a13-/- zebrafish also show an increased rate of occlusive thrombus formation in -/- the caudal venules after FeCl3 injury. More interestingly, a13 zebrafish exhibit ~30% reduction in the number of total, immature, and mature thrombocytes with increased fragmentation of erythrocytes. Administration of a lysine-rich histone results in more severe and persist- Correspondence: ent thrombocytopenia and a significantly increased mortality rate in a13-/- zebrafish than in wildtype (wt) ones. However, both spontaneous and X. -

Evaluation of Clinical and Laboratory Findings of 147 Patients with Systemic Lupus Erythematosus: the Relationship Between Anti-CCP and Arthritis
J Surg Med. 2019;3(3):227-230. Research article DOI: 10.28982/josam.503850 Araştırma makalesi Evaluation of clinical and laboratory findings of 147 patients with systemic lupus erythematosus: The relationship between anti-CCP and arthritis Sistemik lupus eritematozuslu 147 hastanın klinik ve laboratuvar bulgularının değerlendirilmesi: Anti CCP ile artrit arasındaki ilişkinin incelenmesi Ali Ekin 1, Ayşe Ergüney Çefle 2 1 Department of Internal Medicine, Bingol Abstract State Hospital, Bingol, Turkey Aim: Anti-cyclic citrullinated peptide antibodies (Anti-CCP) is considered as a novel marker in the assessment of 2 Department of Internal Medicine, Division of Rheumatology, Faculty of Medicine, Kocaeli rheumatoid disorders. Some studies have emphasized the importance of anti-CCP in indicating erosive arthropathy in University, Kocaeli, Turkey Systemic Lupus Erythematosus (SLE), like Rheumatoid Arthritis (RA). These studies have reported that the chance of erosive arthritis development is significantly increased in anti-CCP-positive patients. This study aimed to investigate ORCID ID of the author(s) the relationship between anti-CCP and arthritis along with other clinical and laboratory parameters in patients with AE: 0000-0003-3692-1293 SLE. AEÇ: 0000-0002-3273-7969 Methods: A total of 147 SLE patients who had been admitted to Kocaeli University Medical Faculty, Department of Internal Medicine, Division of Rheumatology between January 2001 and October 2015 were included in this retrospective study. SLE diagnosis was verified according to American College of Rheumatology (ACR) and/or The Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) criteria. Patients whose diagnosis was not definite and not having anti-CCP were excluded. Results: Female/male ratio was found as 5.6, and the mean age was calculated as 43.9±11.85 years. -

Northwestern Medicine
How to put in your own Photo: Go to ‘View-Slide Master’ Go to ‘Insert-Picture’ Browse to the image you would like to place. Image should be 1024x768, 1200x900, or other 4:3 aspect ratio Select image and click OK Scale to full screen size if To live with lupus, necessary. Click image, go to ‘Format- we need to know Send to Back’ about lupus. Zineb Aouhab, MD Assistant Professor of Medicine Loyola University Medical Center 1 Division of Rheumatology Where did the word “lupus” come from? • The word ‘lupus’ is derived from the Latin word for “wolf”. • Named in the 13th century by the physician “Rogerius”. • He described the rash as erosive facial lesions as a consequence of a wolf's bite. • The facial rash is also called “Butterfly rash” because it resembles a butterfly shape. 2 What is lupus? • Lupus is an autoimmune disease. • It causes chronic inflammation in the body. • It can affect any organ of the body such as the kidneys, gastrointestinal tract, lungs, heart, and joints. 3 What happens in the body? • In normal people, the immune system forms antibodies to fight against infections. • In people with lupus, the immune system become overactive (like a bad allergic reaction) and produces a different types of antibodies. • These antibodies attack the person’s own organs causing organ damage. 4 Factors to consider in lupus • Lupus usually appears between the age of 15-45. • Lupus is much more common in females compared to males. Ratio 9:1 • Lupus is characterized by episodes of flares (when people feel very ill) and periods of remission (when people feel well and back to baseline).