Interpretation Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Autoimmune Pancreatitis: an Autoimmune Or Immunoinflammatory Disease?
10 The Open Autoimmunity Journal, 2011, 3, 10-16 Open Access Autoimmune Pancreatitis: An Autoimmune or Immunoinflammatory Disease? Yvonne Hsieh, Sudhanshu Agrawal, Leman Yel and Sudhir Gupta* Division of Basic and Clinical Immunology, University of California, Irvine, CA 92697, USA Abstract: Autoimmune pancreatitis (AIP) has been widely presumed to be an autoimmune disease that is characterized by elevated IgG and/or IgG4, the presence of autoantibodies, and an infiltration of lymphocytes and plasma cells with fi- brosis. However, no detailed immunological studies have been published. To define immunological changes in AIP in de- tail, and to review evidence for autoimmunity which may be antigen specific and may play a role in the pathogenesis of AIP, and therefore, to determine whether AIP is an autoimmune disease. A detailed immunological investigation for both innate and adaptive immune responses was performed in a patient with AIP. Review of literature was performed from Pub med, and Medline search. Immunological analysis of patient with AIP revealed increased production of proinflammatory IL-6, and IL-17, and increased NK cell activity. No organ-specific or non-specific antibodies were detected. There was no correlation between serum IgG4 with disease activity or response to steroid therapy. Review of literature revealed lack of auto-antigen-specific T and B cell responses in AIP, and autoantibodies are present only in a subset of patients, and are not specific to pancreatic tissue antigens. Therefore, we propose the term Immunoinflammatory pancreatitis rather than an autoimmune pancreatitis. Keywords: NK cells, IL-6, IL-17, autoantibodies, IgG4. INTRODUCTION collectively has been suggested to increase the sensitivity of detection of the disease [6]. -

73 Rheumatoid Factor in Patients with Systemic Lupus Erythematosus
Abstracts Lupus Sci Med: first published as 10.1136/lupus-2019-lsm.73 on 1 April 2019. Downloaded from Conclusions LIT responds appropriately in both directions of the following: LI, anti 2GP1 IgG/IgM, Anti aCL IgG/ to changes in physician (T2T) as well as patient relevant IgM. (DA and health status) outcomes among Spanish SLE Statistical Analysis: Epiinfo version 7.2.0.1. patients. Results We reviewed 147 clinical histories, 107 with RF Funding Source(s): None ordered. Female 93/107 (86.9%), mean age 41.6 years (SD ±13.8). Disease duration 98 months (SD ±80.6). Tobacco exposure 10/107 (9.35%). RF (+) 22/107 (20.5%), RF median titer 228.8 IU/ml (31–2825 IU/ml). – 73 RHEUMATOID FACTOR IN PATIENTS WITH SYSTEMIC RF level in patients with GMN was 142 IU/ml (39 191), – LUPUS ERYTHEMATOSUS without GMN 258 IU/ml (31 2825) p=0.46. RF level in patients with anti Ro (+) was 248 IU/ml (31– Diana G Garate*, Demelza D Yucra, Ruth Balcazar, Hamaui Adriana, Diana Dubinsky. 2825), Ro (-) 52.5 IU/ml (31–74) p=0.37. Sanatorio Guemes RF (-) subgroup showed statistically significant association 10.1136/lupus-2019-lsm.73 with female sex, discoid lesions and aPL presence. RF(+) was associated with Ab Ro, La and patients with RA. (Table 1). Background Rheumatoid factor (RF) in SLE is found in Not found significant association with: rash, photosensivity, around 25% of patients. Authors associate it with cutaneous oral ulcer, joint pain, arthritis, serositis, neurologic and hema- involvement, Sicca, anti Ro (+) and a protective role in glo- tological involvement. -

Domino Effect in a Patient with Epstein-Barr Virus Infection and Autoimmunity: a Case Report Case Report Appointments Scheduled
International Journal of Case Report Clinical Rheumatology Domino effect in a patient with Epstein- Barr Virus infection and autoimmunity: A case report The link between autoimmune diseases and viral infections has been characterized, but specific Margarite Matossian, Carrie mechanisms behind this association remain a current area of investigation. Whether viral infections Crook, Alec Goldberg, Anna trigger or unmask autoimmunity, or if the pathologies occur concurrently, is not yet completely Stathopoulos, Justin Tien, understood. Specifically, Epstein - Barr virus (EBV) is implicated in several autoimmune disorders, Sheela Sheth, Osaid Saqqa & including Systemic Lupus Erythematosus (SLE). It is hypothesized that common immunologic Christopher Dale Shamburger* pathways are activated in the two pathologic states. This case report is an example of this confusing Department of Medicine, Tulane University presentation, and the importance of recognizing the association between autoimmunity and viral Health Sciences Center, New Orleans LA infections. This patient presented with symptoms concerning for SLE and hepatic autoimmunity 70115, USA with serology suggesting a recent infection with EBV. Given this complicated presentation, it is *Author for correspondence: difficult to determine which disease state presented first in patients with evidence of both SLE and EBV infection and whether this information is clinically relevant for ongoing treatment and [email protected] monitoring. Here, we provide an in-depth discussion of current genomic and immunological research that supports the associations amongst these disease pathologies. Introduction by an intricate psychiatric history. Then Autoimmune diseases have increased in we discuss the contribution of infection to frequency in industrialized countries over autoimmune disease states and important recent years [1]. The etiology of autoimmune distinctions between autoimmune diseases diseases, a self-reactive adaptive immune and autoinflammatory responses. -

Pediatric Motor Inflammatory Neuropathy: the Role Of
brain sciences Case Report Pediatric Motor Inflammatory Neuropathy: The Role of Antiphospholipid Antibodies Claudia Brogna 1,2,*, Marco Luigetti 3 , Giulia Norcia 1, Roberta Scalise 1, Gloria Ferrantini 1, Beatrice Berti 1, Domenico M. Romeo 4, Raffaele Manna 5, Eugenio Mercuri 1 and Marika Pane 1 1 Pediatric Neurology and Nemo Clinical Centre, Fondazione Policlinico Universitario A. Gemelli IRCSS, Università Cattolica del Sacro Cuore, 00168 Roma, Italy; [email protected] (G.N.); [email protected] (R.S.); [email protected] (G.F.); [email protected] (B.B.); [email protected] (E.M.); [email protected] (M.P.) 2 Pediatric Neuropsichiatric Unit, ASL, 83100 Avellino, Italy 3 Institute of Neurology, Fondazione Policlinico Universitario A. Gemelli IRCSS, Università Cattolica del sacro Cuore, 00168 Roma, Italy; [email protected] 4 Pediatric Neurology, Fondazione Policlinico Universitario A. Gemelli IRCSS, Università Cattolica del Sacro Cuore, 00168 Roma, Italy; [email protected] 5 Periodic Fevers Research Centre and rare disease. Department of Internal Medicine, Fondazione Policlinico Universitario A. Gemelli IRCSS, Università Cattolica del Sacro Cuore, 00168 Roma, Italy; raff[email protected] * Correspondence: [email protected] Received: 4 February 2020; Accepted: 5 March 2020; Published: 7 March 2020 Abstract: We report the clinical case of a nine-year-old girl who presented with progressive motor neuropathy, revealed via the detection of a higher delay in F-wave recording using digitalized nerve conduction/electromyography. Since the lupus anticoagulant (LAC) positivity, detected using diluted Russell viper venom time (dRVVT), switched to persistent serological anticardiolipin immunoglobulin G (IgG) positivity, a possible non-thrombotic antiphospholipid antibody (aPL)-related clinical manifestation was suspected, and intravenous immunoglobulin treatment (IVIG) was started. -
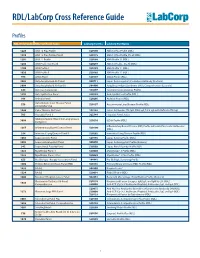
RDL/Labcorp Cross Reference Guide
RDL/LabCorp Cross Reference Guide Profiles RDL Order Code RDL Test/Panel Name LabCorp Test No. LabCorp Test Name 1228 ANA 12 Plus Profile 520180 ANA 12 Plus Profile (RDL) 1230 ANA 12 Plus Profile, Do All 520175 ANA 12 Plus Profile, Do all (RDL) 1201 ANA 12 Profile 520188 ANA Profile 12 (RDL) 1206 ANA Profile 12, Do All 520299 ANA 12 Profile , Do All (RDL) 1100 ANA Profile I 520185 ANA Profile 11 (RDL) 1020 ANA Profile II 520185 ANA Profile 11 (RDL) 994 ANCA Panel 520149 ANCA Profile (RDL) 3003 Antiphospholipid Ab Panel I 500711 Lupus Anticoagulant/Cardiolipin Antibody (Esoterix) 3004 Antiphospholipid Ab Panel II 504400 Antiphospholipid Syndrome (APS), Comprehensive (Esoterix) 644 Anti-Saccharomyces 164657 Saccharomyces cerevisiae Profile 1292 Anti-Synthetase Panel 520193 Anti-Synthetase Profile (RDL) 996 Arthritis Panel 520205 Arthritis Profile (RDL) Autoimmune Liver Disease Panel, 558 520197 Autoimmune Liver Disease Profile (RDL) Comprehensive 1044 Celiac Disease Ab Panel 165142 Celiac Antibodies tTG IgA, EMA IgA, Total IgA with Reflex to tTG IgG 705 Hepatitis Panel II 322744 Hepatitis Panel, Acute ILDdxComplete (Interstitial Lung Disease 3008 520210 ILDdx Profile (RDL) Complete) Inflammatory Bowel Disease (IBD) Profile with Anti-Pancreatic Antibodies 1265 Inflammatory Bowel Disease Panel 520190 (RDL) 354 Interstitial Lung Disease Panel II 520202 Interstitial Lung Disease Profile (RDL) 1065 Lupus Activity Panel 520195 Lupus Activity Profile (RDL) 3002 Lupus Anticoagulant Panel 500070 Lupus Anticoagulant Profile (Esoterix) 342 Lupus Renal -

32 Mechanism of Thrombosis in Antiphospholipid Syndrome: Binding to Platelets Joan-Carles Reverter and Dolors Tàssies
32 Mechanism of Thrombosis in Antiphospholipid Syndrome: Binding to Platelets Joan-Carles Reverter and Dolors Tàssies Introduction Antiphospholipid antibodies (aPL) are related to thrombosis in the antiphospho- lipid syndrome (APS) [1, 2] and numerous pathophysiological mechanisms have been suggested involving cellular effects, plasma coagulation regulatory proteins, and fibrinolysis [3, 4]: aPL may act as blocking agents directly inhibiting antigen enzymatic or co-factor function of hemostasis; may bind fluid-phase antigens of hemostasis involved proteins and then decrease plasma antigen levels by clearance of immune complexes; may form immune complexes with their antigens that may be deposited in blood vessels causing inflammation and tissue injury; may cause dysregulation of antigen–phospholipid binding due to cross-linking of membrane bound antigens; and may trigger cell mediated events by cross-linking of antigen bound to cell surfaces or cell surface receptors [3, 4]. Moreover, several characteris- tics of the aPL, such as the concentration, class/subclass, affinity or charge, and several characteristics of the antigens, as the concentration, size, location or charge, may influence which of the theoretical autoantibody actions will occur in vivo [3]. Among the cellular mechanisms supposed to be involved, platelets have been considered as one of the most promising potential target for circulating aPL that may cause antibody mediated thrombosis as a part of the clinical spectrum of the autoimmune disorder of the APS. In the present chapter we will focus on the inter- actions that involve aPL binding to platelet membrane or platelet membrane bound antigens. Platelets as Target for aPL Platelets play a central role in primary hemostasis involving platelet adhesion to the injured blood vessel wall, followed by platelet activation, granule release, shape change, and rearrangement of the outer membrane phospholipids and proteins, transforming them into a highly efficient procoagulant surface [5]. -

Rheumatoid Factor: a Novel Determiner in Cancer History
cancers Commentary Rheumatoid Factor: A Novel Determiner in Cancer History Alessio Ugolini 1,2 and Marianna Nuti 1,* 1 Department of Experimental Medicine, “Sapienza” University of Rome, Viale Regina Elena 324, 00161 Rome, Italy; alessio.ugolini@moffitt.org 2 Department of Immunology, H. Lee Moffitt Cancer Center, Tampa, FL 33612, USA * Correspondence: [email protected] Simple Summary: Rheumatoid factors are autoantibodies that characterize different autoimmune diseases, in particular rheumatoid arthritis, but that can also be found in the sera of the general healthy population. They have been mainly studied in the context of autoimmune diseases, but some evidence have suggested an association between their presence and the predisposition to develop cancer as well as a facilitation of cancer growth and progression in oncologic patients. In this review, for the first time we thus analyze and discuss the possible roles that these autoantibodies can assume in tumor history, from determiners of a heightened susceptibility of developing cancer to drivers of a reduced response to immunotherapies. Abstract: The possible interplay between autoimmunity and cancer is a topic that still needs to be deeply explored. Rheumatoid factors are autoantibodies that are able to bind the constant regions (Fc) of immunoglobulins class G (IgGs). In physiological conditions, their production is a transient event aimed at contributing to the elimination of pathogens as well as limiting a redundant immune response by facilitating the clearance of antibodies and immune complexes. Their production can become persistent in case of different chronic infections or diseases, being for instance a fundamental marker for the diagnosis and prognosis of rheumatoid arthritis. -

Antisynthetase Syndrome and Rheumatoid Arthritis: a Rare Overlapping Disease
Case Report Annals of Clinical Case Reports Published: 15 Jul, 2020 Antisynthetase Syndrome and Rheumatoid Arthritis: A Rare Overlapping Disease Makhlouf Yasmine*, Miladi Saoussen, Fazaa Alia, Sallemi Mariem, Ouenniche Kmar, Leila Souebni, Kassab Selma, Chekili Selma, Zakraoui Leith, Ben Abdelghani Kawther and Laatar Ahmed Department of Rheumatology, Mongi Slim Hospital, Tunisia Abstract The association between Antisynthetase Syndrome (ASS) and rheumatoid arthritis is extremely rare. In this case report, we are describing a 16 years long standing history of seropositive RA before its uncommon association to an ASS. A 55-year-old female patient presented at the first visit with symmetric polyarthritis and active synovitis affecting both hands and ankles. Laboratory investigations showed positive rheumatoid factors, positive anti-CCP antibodies and negative ANA. The X-rays were consistent with typical erosive in hands. Thus, the patient fulfilled the ACR 1987 criteria of RA in 2000 at the age of 37. Methotrexate was firstly prescribed. However, it was ineffective after 4 years. Then, the patient did well with Leflunomide until January 2017, when she developed exertional dyspnea. High-resolution CT of the lung revealed Nonspecific Interstitial Pneumonia (NSIP). Autoantibodies against extractable nuclear antigens were screened and showed positive results for anti-Jo1 autoantibodies. She was diagnosed with ASS complicating the course of RA. Keywords: Antisynthetase syndrome; Rheumatoid arthritis; Overlap syndrome; Nonspecific interstitial pneumonia Key Points • Antisynthetase Syndrome should be considered as a clinical manifestation of overlap syndromes, particularly in active RA patients with pulmonary signs and anti-Jo-1 antibody. OPEN ACCESS • An early diagnosis of antisynthetase syndrome in an overlap syndrome is important, as *Correspondence: treatment may need adjustment. -

Significant Factors Associated with Lupus Nephritis P Alba, L Bento, M J Cuadrado, Y Karim, M F Tungekar, I Abbs, M a Khamashta, D D’Cruz,Grvhughes
556 EXTENDED REPORT Ann Rheum Dis: first published as 10.1136/ard.62.6.556 on 1 June 2003. Downloaded from Anti-dsDNA, anti-Sm antibodies, and the lupus anticoagulant: significant factors associated with lupus nephritis P Alba, L Bento, M J Cuadrado, Y Karim, M F Tungekar, I Abbs, M A Khamashta, D D’Cruz,GRVHughes ............................................................................................................................. Ann Rheum Dis 2003;62:556–560 Background: Lupus nephritis (LN) is a common manifestation in patients with systemic lupus erythema- tosus (SLE). Autoantibodies and ethnicity have been associated with LN, but the results are controver- sial. Objective: To study the immunological and demographic factors associated with the development of LN. Patients and methods: A retrospective case-control study of 127 patients with biopsy-proven LN, and 206 randomly selected patients with SLE without nephritis as controls was designed. All patients had attended our lupus unit during the past 12 years. Standard methods were used for laboratory testing. Results: Patients with LN were significantly younger than the controls at the time of SLE diagnosis See end of article for (mean (SD) 25.6 (8.8) years v 33.7 (12.5) years; p<0.0001). The proportion of patients of black eth- authors’ affiliations nic origin was significantly higher in the group with nephritis (p=0.02). There were no differences in ....................... sex distribution or duration of follow up. A higher proportion of anti-dsDNA, anti-RNP, anti-Sm, and Correspondence to: lupus anticoagulant (LA) was seen in the group with nephritis (p=0.002; p=0.005; p=0.0001; Dr M J Cuadrado, Lupus p=0.01, respectively). -

Hepatic Manifestations of the Antiphospholipid Syndrome
EDITORIAL Hepatic Manifestations of the Antiphospholipid Syndrome Key words: autoimmune hepatitis, Sjogren's syndrome, Budd- autoimmune hepatitis (7). Examinations prior to liver biopsy Chiari syndrome, nodular regenerative hyperpla- revealed a greatly prolonged APTT,and the patient was found sia to be positive for IgG anticardiolipin antibody and lupus anti- coagulant. However, this patient showedno signs of thrombo- sis or any other symptomssuggestive ofAPS. In this issue, Katayama et al (8) report a case of Sjogren's Antiphospholipid syndrome (APS) is characterized by the syndrome complicated with autoimmune hepatitis and APS. presence of antiphospholipid antibodies (anticardiolipin anti- bodies and/or lupus anticoagulant) in association with symp- See also p 73. toms including venous/arterial thromboses, recurrent fetal loss and thrombocytopenia. Thromboticepisodes mayoccur at any In previously reported cases of autoimmune hepatitis asso- part of the circulation system, hence, any organ may be in- ciated with the presence of antiphospholipid antibodies, anti- volved. As for hepatic involvement, the most well knownmani- nuclear antibody was present, but the presence of anti-SS-A festation ofAPSis Budd-Chiari syndrome, which is caused antibody or sicca symptomshave not been documented. There- mostly by obstruction of hepatic veins or inferior vena cava. fore, this case may possibly be the first documented case of Pelletier et al (1) have reported that of their 22 non-tumourous autoimmune hepatitis with APSand Sjogren's syndrome. Their Budd-Chiari patients, 4 had antiphospholipid antibodies with- patient had autoimmune hepatitis, and Sjogren's syndrome out other causes of hepatic vein thrombosis. Amongthese 4 confirmed by Schirmer's test and sialography. The patient patients, 2 seemed to have primary APS, i.e., no signs of other showed positive for (32-GPI dependent anticardiolipin antibody autoimmunediseases such as SLE. -

Evaluation of Clinical and Laboratory Findings of 147 Patients with Systemic Lupus Erythematosus: the Relationship Between Anti-CCP and Arthritis
J Surg Med. 2019;3(3):227-230. Research article DOI: 10.28982/josam.503850 Araştırma makalesi Evaluation of clinical and laboratory findings of 147 patients with systemic lupus erythematosus: The relationship between anti-CCP and arthritis Sistemik lupus eritematozuslu 147 hastanın klinik ve laboratuvar bulgularının değerlendirilmesi: Anti CCP ile artrit arasındaki ilişkinin incelenmesi Ali Ekin 1, Ayşe Ergüney Çefle 2 1 Department of Internal Medicine, Bingol Abstract State Hospital, Bingol, Turkey Aim: Anti-cyclic citrullinated peptide antibodies (Anti-CCP) is considered as a novel marker in the assessment of 2 Department of Internal Medicine, Division of Rheumatology, Faculty of Medicine, Kocaeli rheumatoid disorders. Some studies have emphasized the importance of anti-CCP in indicating erosive arthropathy in University, Kocaeli, Turkey Systemic Lupus Erythematosus (SLE), like Rheumatoid Arthritis (RA). These studies have reported that the chance of erosive arthritis development is significantly increased in anti-CCP-positive patients. This study aimed to investigate ORCID ID of the author(s) the relationship between anti-CCP and arthritis along with other clinical and laboratory parameters in patients with AE: 0000-0003-3692-1293 SLE. AEÇ: 0000-0002-3273-7969 Methods: A total of 147 SLE patients who had been admitted to Kocaeli University Medical Faculty, Department of Internal Medicine, Division of Rheumatology between January 2001 and October 2015 were included in this retrospective study. SLE diagnosis was verified according to American College of Rheumatology (ACR) and/or The Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) criteria. Patients whose diagnosis was not definite and not having anti-CCP were excluded. Results: Female/male ratio was found as 5.6, and the mean age was calculated as 43.9±11.85 years. -

What Should I Do with a Positive ANA Or RF?
What should I do with a positive ANA or RF? ACP Puerto Rico Chapter Meeting March 13-14, 2020 Elena Myasoedova, MD, PhD Associate Professor of Medicine Mayo Clinic Rochester, Minnesota [email protected] ©2017 MFMER | slide-1 Disclosures • None ©2017 MFMER | slide-2 Why is this important? • Patients are frequently tested for RF or ANA without specific indication • Patients are referred to Rheumatology clinic on the basis of positive RF/ ANA • Patients and primary care providers are often uncertain regarding the meaning of abnormal test ©2017 MFMER | slide-3 Learning objectives • Understand the circumstances when ordering RF or ANA is helpful • Learn how to interpret a positive RF or ANA • Understand the indications for referral to Rheumatology in patients with positive RF or ANA ©2017 MFMER | slide-4 Laboratory Case 1 • You are asked to evaluate a 74 year old woman who was found to have a positive RF of 84 IU/ml by her orthopedic surgeon. The test was obtained to evaluate multiple chronic joint pains in her hands and knees. She is otherwise well and takes OTC naproxen for her pains. Her ESR is 24 mm/ hour • Your examination reveals nodular osteoarthritis of the fingers, bilateral genu varus deformities of the knees with a small, cool effusion on the right. ©2017 MFMER | slide-5 Laboratory Case 3 • You conclude that she has generalized osteoarthritis, no signs of RA and that the positive RF is likely age related. • Which one of the following tests could you order to further support your opinion? 1. C-reactive protein 2.