©Ferrata Storti Foundation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Mutational Landscape of Myeloid Leukaemia in Down Syndrome
cancers Review The Mutational Landscape of Myeloid Leukaemia in Down Syndrome Carini Picardi Morais de Castro 1, Maria Cadefau 1,2 and Sergi Cuartero 1,2,* 1 Josep Carreras Leukaemia Research Institute (IJC), Campus Can Ruti, 08916 Badalona, Spain; [email protected] (C.P.M.d.C); [email protected] (M.C.) 2 Germans Trias i Pujol Research Institute (IGTP), Campus Can Ruti, 08916 Badalona, Spain * Correspondence: [email protected] Simple Summary: Leukaemia occurs when specific mutations promote aberrant transcriptional and proliferation programs, which drive uncontrolled cell division and inhibit the cell’s capacity to differentiate. In this review, we summarize the most frequent genetic lesions found in myeloid leukaemia of Down syndrome, a rare paediatric leukaemia specific to individuals with trisomy 21. The evolution of this disease follows a well-defined sequence of events and represents a unique model to understand how the ordered acquisition of mutations drives malignancy. Abstract: Children with Down syndrome (DS) are particularly prone to haematopoietic disorders. Paediatric myeloid malignancies in DS occur at an unusually high frequency and generally follow a well-defined stepwise clinical evolution. First, the acquisition of mutations in the GATA1 transcription factor gives rise to a transient myeloproliferative disorder (TMD) in DS newborns. While this condition spontaneously resolves in most cases, some clones can acquire additional mutations, which trigger myeloid leukaemia of Down syndrome (ML-DS). These secondary mutations are predominantly found in chromatin and epigenetic regulators—such as cohesin, CTCF or EZH2—and Citation: de Castro, C.P.M.; Cadefau, in signalling mediators of the JAK/STAT and RAS pathways. -

Adult Acute Myeloid Leukemia with Trisomy 11 As the Sole Abnormality
Letters to the Editor 2254 Adult acute myeloid leukemia with trisomy 11 as the sole abnormality is characterized by the presence of five distinct gene mutations: MLL-PTD, DNMT3A, U2AF1, FLT3-ITD and IDH2 Leukemia (2016) 30, 2254–2258; doi:10.1038/leu.2016.196 sequencing approach at the DNA level were also analyzed at the RNA level by visual inspection of the BAM files. The clinical characteristics and outcomes of 23 patients with Trisomy of chromosome 11 (+11) is the second most common sole +11 are summarized in Table 1. The patients were isolated trisomy in acute myeloid leukemia (AML) patients.1 The presence of +11 is associated with intermediate2,3 or poor 4–6 Table 1. Pretreatment clinical and molecular characteristics and patient outcomes. Whereas the clinical characteristics of solitary outcome of patients with acute myeloid leukemia (AML) and sole +11 +11 have been well established,4–6 relatively little is known about the mutational landscape of sole +11 AML in the age of next- Characteristica Sole +11 AML (n = 23) generation sequencing techniques that allow examination of multiple genes relevant to AML pathogenesis.6 So far, the most Age, years Median 71 common molecular feature in AML with isolated +11 is the – presence of a partial tandem duplication of the MLL (KMT2A) gene Range 25 84 (MLL-PTD), which is detectable in up to 90% of patients.7 Age group, n (%) Furthermore, a frequent co-occurrence of the FLT3 internal o60 years 18 (78) tandem duplication (FLT3-ITD) with MLL-PTD has been reported.8 ⩾ 60 years 5 (22) The aim of our study was to better characterize the mutational Female sex, n (%) 5 (22) landscape of adult AML patients with sole +11. -

ABC of Clinical Genetics CHROMOSOMAL DISORDERS II
ABC of Clinical Genetics CHROMOSOMAL DISORDERS II BMJ: first published as 10.1136/bmj.298.6676.813 on 25 March 1989. Downloaded from Helen M Kingston Developmental delay in Chromosomal abnormalities are generally associated with multiple child with deletion of congenital malformations and mental retardation. Children with more than chromosome 13. one physical abnormality, particularly ifretarded, should therefore undergo chromosomal analysis as part of their investigation. Chromosomal disorders are incurable but can be reliably detected by prenatal diagnostic techniques. Amniocentesis or chorionic villus sampling should be offered to women whose pregnancies are at increased risk-namely, women in their mid to late thirties, couples with an affected child, and couples in whom one partner carries a balanced translocation. Unfortunately, when there is no history of previous abnormality the risk in many affected pregnancies cannot be predicted beforehand. Autosomal abnormalities Parents Non-dysjunction Trisomy 21 (Down's syndrome) Down's syndrome is the commonest autosomal Gametes trisomy, the incidence in liveborn infants being one in 650, although more than half of conceptions with trisomy 21 do not survive to term. Affected children have a characteristic Offspring facial appearance, are mentally retarded, and Trisomy 21 often die young. They may have associated Non-dysjunction of chromosome 21 leading to Down's syndrome. congenital heart disease and are at increased risk recurrent for infections, atlantoaxial instability, http://www.bmj.com/ -- All chromosomal abnormalities at and acute leukaemia. They are often happy and 100 - ainniocentesis ---- Downl's syndrome at amniocentesis Risk for trisomy 21 in liveborn infants affectionate children who are easy to manage. -

Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Down Syndrome
COMMON TYPES OF CHROMOSOME ABNORMALITIES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA A. Trisomy: instead of having the normal two copies of each chromosome, an individual has three of a particular chromosome. Which chromosome is trisomic determines the type and severity of the disorder. Down syndrome or Trisomy 21, is the most common trisomy, occurring in 1 per 800 births (about 3,400) a year in the United States. It is one of the most common genetic birth defects. According to the National Down Syndrome Society, there are more than 400,000 individuals with Down syndrome in the United States. Patients with Down syndrome have three copies of their 21 chromosomes instead of the normal two. The major clinical features of Down syndrome patients include low muscle tone, small stature, an upward slant to the eyes, a single deep crease across the center of the palm, mental retardation, and physical abnormalities, including heart and intestinal defects, and increased risk of leukemia. Every person with Down syndrome is a unique individual and may possess these characteristics to different degrees. Down syndrome patients Karyotype of a male patient with trisomy 21 What are the causes of Down syndrome? • 95% of all Down syndrome patients have a trisomy due to nondisjunction before fertilization • 1-2% have a mosaic karyotype due to nondisjunction after fertilization • 3-4% are due to a translocation 1. Nondisjunction refers to the failure of chromosomes to separate during cell division in the formation of the egg, sperm, or the fetus, causing an abnormal number of chromosomes. As a result, the baby may have an extra chromosome (trisomy). -

Cytogenetics, Chromosomal Genetics
Cytogenetics Chromosomal Genetics Sophie Dahoun Service de Génétique Médicale, HUG Geneva, Switzerland [email protected] Training Course in Sexual and Reproductive Health Research Geneva 2010 Cytogenetics is the branch of genetics that correlates the structure, number, and behaviour of chromosomes with heredity and diseases Conventional cytogenetics Molecular cytogenetics Molecular Biology I. Karyotype Definition Chromosomal Banding Resolution limits Nomenclature The metaphasic chromosome telomeres p arm q arm G-banded Human Karyotype Tjio & Levan 1956 Karyotype: The characterization of the chromosomal complement of an individual's cell, including number, form, and size of the chromosomes. A photomicrograph of chromosomes arranged according to a standard classification. A chromosome banding pattern is comprised of alternating light and dark stripes, or bands, that appear along its length after being stained with a dye. A unique banding pattern is used to identify each chromosome Chromosome banding techniques and staining Giemsa has become the most commonly used stain in cytogenetic analysis. Most G-banding techniques require pretreating the chromosomes with a proteolytic enzyme such as trypsin. G- banding preferentially stains the regions of DNA that are rich in adenine and thymine. R-banding involves pretreating cells with a hot salt solution that denatures DNA that is rich in adenine and thymine. The chromosomes are then stained with Giemsa. C-banding stains areas of heterochromatin, which are tightly packed and contain -

Paternally Inherited Trisomy at D21S11 and Mutation at DXS10135 Microsatellite Marker in a Case of Fetus Paternity Establishment
Egyptian Journal of Forensic Sciences (2015) xxx, xxx–xxx HOSTED BY Contents lists available at ScienceDirect Egyptian Journal of Forensic Sciences journal homepage: http://www.journals.elsevier.com/egyptian-journal-of-forensic-sciences CASE REPORT Paternally inherited trisomy at D21S11 and mutation at DXS10135 microsatellite marker in a case of fetus paternity establishment Pankaj Shrivastava a,*, Toshi Jain a,b, Sonia Kakkar c, Veena Ben Trivedi a a DNA Fingerprinting Unit, State Forensic Science Laboratory, Department of Home (Police), Govt. of Madhya Pradesh, Sagar 470001, MP, India b School of Studies in Microbiology, Jiwaji University, Gwalior, MP, India c Deptt of Forensic Medicine, PGIMER, Chandigarh, India Received 3 June 2015; revised 29 July 2015; accepted 6 September 2015 KEYWORDS Abstract The case of establishment of paternity of an aborted fetus was examined with 15 auto- DNA profiling; somal STR markers. The genotype of the fetus was X at amelogenin marker and showed inheritance D21S11; of both the alleles of father at D21S11 marker, thus displaying unusual tri-allelic pattern. The cases DXS10135; where mutation in any of biparental autosomal STR markers is observed, the use of additional STR X-STR; marker system is recommended. On testing all the three samples with 12 X-STR markers, all the Trisomy; maternal and paternal alleles were accounted in the female fetus except at DXS10135 marker. Mutation The genotypes of mother, fetus and father at DXS10135 were 20, 22; 20, 20 and 21, which confirmed mutation of the paternal allele in the female fetus. The paternal allele contracted from 21 to 20. The allele peak heights of D21S11 and DXS10135 markers were also examined to rule out the possibility of any false allele. -

NIPT Fact Sheet
Victorian Clinical Genetics Services Murdoch Childrens Research Institute The Royal Children's Hospital Flemington Road, Parkville VIC 3052 P (03) 8341 6201 W vcgs.org.au NIPT fact sheet High risk for XYY This fact sheet is for women and their partners who receive a high Possible explanations for this risk result for XYY from the perceptTM non-invasive prenatal test (NIPT) offered by Victorian Clinical Genetics Services (VCGS). This information high risk result: may not be applicable to test results reported by other NIPT service There is a high chance that the baby has XYY. providers. However, the only way to provide a definitive As part of the service offered by VCGS, women and couples have diagnosis is to have a diagnostic procedure the opportunity to discuss their result with a genetic counsellor at no (CVS or amniocentesis) with chromosome additional cost. Genetic counsellors are experts in assisting people to testing. understand genetic test results and make informed decisions about In some cases, high risk results for XYY may further testing options. represent ‘false positive’ test results. A false positive result means that although NIPT indicates a high risk of XYY, the baby does not What does this result mean? have this condition. NIPT is a way for women to get an accurate estimate of the chance that their baby has one of the most common chromosome conditions: Possible causes of false positive results trisomy 21 (Down syndrome), trisomy 18 and trisomy 13. The test for XYY from NIPT include: may also detect whether there are extra or missing copies of the sex chromosomes, X and Y. -

Trisomy 8 Mosaicism
Trisomy 8 Mosaicism rarechromo.org Sources Trisomy 8 Mosaicism Trisomy 8 mosaicism (T8M) is a chromosome disorder caused by and the presence of a complete extra chromosome 8 in some cells of References the body. The remaining cells have the usual number of 46 The information in chromosomes, with two copies of chromosome 8 in each cell. this guide is Occasionally T8M is called Warkany syndrome after Dr Josef drawn partly from Warkany, the American paediatrician who first identified the published medical condition and its cause in the 1960s. Full trisomy 8 – where all cells literature. The have an extra copy of chromosome 8 - is believed to be incompatible first-named with survival, so babies and children in whom an extra chromosome author and 8 is found are believed to be always mosaic (Berry 1978; Chandley publication date 1980; Jordan 1998; Karadima 1998). are given to allow you to look for the Genes and chromosomes abstracts or The human body is made up of trillions of cells. Most of the cells original articles contain a set of around 20,000 different genes; this genetic on the internet in information tells the body how to develop, grow and function. Genes PubMed are carried on structures called chromosomes, which carry the (www.ncbi.nlm. genetic material, or DNA, that makes up our genes. nih.gov/pubmed). If you wish, you Chromosomes usually come in pairs: one chromosome from each can obtain most parent. Of these 46 chromosomes, two are a pair of sex articles from chromosomes: XX (a pair of X chromosomes) in females, and XY Unique. -
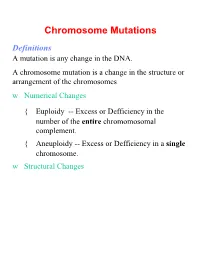
Chromosome Mutations Definitions a Mutation Is Any Change in the DNA
Chromosome Mutations Definitions A mutation is any change in the DNA. A chromosome mutation is a change in the structure or arrangement of the chromosomes w Numerical Changes { Euploidy -- Excess or Defficiency in the number of the entire chromomosomal complement. { Aneuploidy -- Excess or Defficiency in a single chromosome. w Structural Changes Numerical Chromosome Mutations Euploid -- “True Number” The cell(s) have the same number of copies of all the chromosome. 1. Monoploid (Haploid if a gamete) 2. Diploid (Often is not considered a Euploid) 3. Triploid 4. Tetraploid Euploidy Chromosomes shown at metaphase Normal Diploid Euploid Monoploid Triploid Tetraploid Euploidy is an excess or deficiency for the entire complement of chromosomes. Euploidy Organisms w Plants, amphibians, lower reptiles w Some cells in other organisms (liver, midgut, etc.) Root Causes w Nondisjunction w Autoreduplication Euploidy (continued) Origins w Autopolyploidy { Often involving individuals within the same species. { Infertile. Propagation by cloning. w Allopolyploidy { Often involving individuals from different species. { Fertile. Dosage Concerns Examples Numerical Chromosome Mutations Aneuploidy Excess or Defficiency in a single chromosome. 1. Monosomy 2. Disomy (only if normally cell is not diploid) 3. Trisomy 4. Tetrasomy Aneuploidy Chromosomes shown at metaphase Normal Diploid Aneuploid Monosomic Trisomic Tetrasomic Aneuploidy is an excess or deficiency for fewer than the entire complement of chromosomes. Aneuploidy Organisms w All organisms w Variability in how they deal with it Root Causes w Nondisjunction w Results of Chromosome Structure Mutations Aneuploidy -- Examples Plants w Jimson Weed (Datura stramonium) Animals w Drosophila: Triploid females are viable & fertile. Trisomy flies are not! w Human (autosomal): { Trisomy 13 (Patau Syndrome) 1:20,000 { Trisomy 18 (Edwards Syndrome) 1:10,000 { Trisomy 21 (Down Syndrome) 1:700 { Other rare ones (7, 9, 12, 14, & 22) Aneuploidy Examples w Human Sex Determining Chromosomes 1. -

Chromosomal Disorders
Understanding Genetic Tests and How They Are Used David Flannery,MD Medical Director American College of Medical Genetics and Genomics Starting Points • Genes are made of DNA and are carried on chromosomes • Genetic disorders are the result of alteration of genetic material • These changes may or may not be inherited Objectives • To explain what variety of genetic tests are now available • What these tests entail • What the different tests can detect • How to decide which test(s) is appropriate for a given clinical situation Types of Genetic Tests . Cytogenetic . (Chromosomes) . DNA . Metabolic . (Biochemical) Chromosome Test (Karyotype) How a Chromosome test is Performed Medicaldictionary.com Use of Karyotype http://medgen.genetics.utah.e du/photographs/diseases/high /peri001.jpg Karyotype Detects Various Chromosome Abnormalities • Aneuploidy- to many or to few chromosomes – Trisomy, Monosomy, etc. • Deletions – missing part of a chromosome – Partial monosomy • Duplications – extra parts of chromosomes – Partial trisomy • Translocations – Balanced or unbalanced Karyotyping has its Limits • Many deletions or duplications that are clinically significant are not visible on high-resolution karyotyping • These are called “microdeletions” or “microduplications” Microdeletions or microduplications are detected by FISH test • Fluorescence In situ Hybridization FISH fluorescent in situ hybridization: (FISH) A technique used to identify the presence of specific chromosomes or chromosomal regions through hybridization (attachment) of fluorescently-labeled DNA probes to denatured chromosomal DNA. Step 1. Preparation of probe. A probe is a fluorescently-labeled segment of DNA comlementary to a chromosomal region of interest. Step 2. Hybridization. Denatured chromosomes fixed on a microscope slide are exposed to the fluorescently-labeled probe. Hybridization (attachment) occurs between the probe and complementary (i.e., matching) chromosomal DNA. -

NIPT Fact Sheet
Victorian Clinical Genetics Services Murdoch Childrens Research Institute The Royal Children's Hospital Flemington Road, Parkville VIC 3052 P (03) 8341 6201 W vcgs.org.au NIPT fact sheet High risk for Monosomy X This fact sheet is for women and their partners who receive a high risk Possible explanations for this result for monosomy X from the perceptTM non-invasive prenatal test (NIPT) offered by the Victorian Clinical Genetics Services (VCGS). This high risk result: information may not be applicable to test results reported by other NIPT There is a chance that the baby has monosomy service providers. X however, only about 1 in 5 (or 20%) high risk As part of the service offered by VCGS, women and their partners have monosomy X results are associated with a true the opportunity to discuss their result with a genetic counsellor at no finding of monosomy X in the baby. additional cost. Genetic counsellors are experts in assisting people to The only way to provide a definitive diagnosis understand genetic test results and make informed decisions about is to have a diagnostic procedure (CVS or further testing options. amniocentesis) with chromosome testing. High risk results for monosomy X are often ‘false positive’ test results. A false positive result What does this result mean? means that although NIPT indicates a high risk NIPT is a way for women to get an accurate estimate of the chance of monosomy X, the baby does not have this that their baby has one of the most common chromosome conditions: condition. trisomy 21 (Down syndrome), trisomy 18 and trisomy 13. -

Trisomy 13 (Patau Syndrome)
General information about positive NIPT results What testing could be considered? My patient’s NIPT is positive for 13 (Patau syndrome). What does this mean? Your patient’s NIPT result suggests • Specialized genetic tests such as karyotyping, fluorescence the presence of an extra copy of chromosome 13. NIPT is a in situ hybridization (FISH), qPCR, and microarray are available screening test; false positives can occur. The actual chance for to confirm the presence of trisomy 13. the pregnancy to have trisomy 13 depends on many factors, • These confirmatory tests are generally performed on cells from including the patient’s clinical and family history. chorionic villus sampling (CVS) or amniocentesis during Next steps to consider: You should discuss the results pregnancy, on cord blood or peripheral blood sample after the and the potential clinical implications with your patient. The baby is born, or on products of conception (POC) in case of American College of Obstetricians and Gynecologists and the a miscarriage. Society for Maternal-Fetal Medicine state, “All women with a • Ultrasound evaluation may be useful in aiding with a prenatal positive cell-free DNA test result should have further detailed diagnosis of trisomy 13, but a normal ultrasound cannot exclude counseling and testing and should have a diagnostic procedure this condition. before any irreversible action is taken.”1 Confirmation prior to birth can also help with pregnancy and neonatal management. Resources for trisomy 13: Please see below for more information about trisomy 13 Genetics Home Reference http://ghr.nlm.nih.gov/chromosome/13 and additional resources. Unique, The Rare Chromosome Disorder Support Group http://www.rarechromo.org/ What is trisomy 13? Trisomy 13 is a condition that is caused by an extra chromosome number 13 (three copies instead of two).