Potential of Polyphenols As Therapeutic Options in Type 2 Diabetes Elham Nikbakht
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluating the Agricultural, Historical, Nutritional, and Sustainable Uses of Pulse Grains and Legumes
EVALUATING THE AGRICULTURAL, HISTORICAL, NUTRITIONAL, AND SUSTAINABLE USES OF PULSE GRAINS AND LEGUMES A THESIS SUBMITTED TO THE FACULTY OF UNIVERSITY OF MINNESOTA BY Stefanie Marie Havemeier IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE June 2018 ©Stefanie Marie Havemeier 2018 Acknowledgements First and foremost, I would like to extend my sincerest appreciation to my advisor, Dr. Joanne Slavin, for her guidance, trust, and support throughout my graduate degree. She is a role model to all, especially her graduate students, and her positive attitude brings life to any arduous task. I would undoubtedly not be where I am today if it were not for Dr. Slavin providing me with the opportunity to work alongside her. I would also like to thank my other advisory committee members: Dr. Dave Smith and Dr. Renee Korczak. Thank you, Dr. Dave Smith, for providing me with fundamental information that forms the basis of food science and always a good laugh in the classroom. Thank you, Dr. Korczak, for allowing me to work beside you as your teacher’s assistant, barreling through endless student emails together. I thank my lab mates, Alexis, Hannah, Jennifer, Julie, Justin, and Rylee, for providing guidance and advice and for always listening. I would not have been able to complete this journey without your constant support. To my parents, David and Jeane, I would like to thank you for your encouragement and unending support not only throughout this process but, throughout my entire life. To my sister, Stacie, thank you for listening to me talk, “about my beans,” endlessly. -

Research Online
University of Wollongong Research Online Faculty of Science, Medicine and Health - Papers: part A Faculty of Science, Medicine and Health 1-1-2014 Food patterns of Australian children ages 9 to 13 y in relation to ω-3 long chain polyunsaturated intake Setyaningrum Rahmawaty University of Wollongong Philippa Lyons-Wall Edith Cowan University, [email protected] Marijka Batterham University of Wollongong, [email protected] Karen Charlton University of Wollongong, [email protected] Barbara J. Meyer University of Wollongong, [email protected] Follow this and additional works at: https://ro.uow.edu.au/smhpapers Part of the Medicine and Health Sciences Commons, and the Social and Behavioral Sciences Commons Recommended Citation Rahmawaty, Setyaningrum; Lyons-Wall, Philippa; Batterham, Marijka; Charlton, Karen; and Meyer, Barbara J., "Food patterns of Australian children ages 9 to 13 y in relation to ω-3 long chain polyunsaturated intake" (2014). Faculty of Science, Medicine and Health - Papers: part A. 1347. https://ro.uow.edu.au/smhpapers/1347 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] Food patterns of Australian children ages 9 to 13 y in relation to ω-3 long chain polyunsaturated intake Abstract Objective: The aim of this study was to examine food patterns of Australian children ages 9 to 13 y in relation to ω-3 long-chain polyunsaturated fatty acid (ω-3 LCPUFA) intake. Methods: Secondary analysis was conducted on nationally representative food data of 1110 Australian children ages 9 to 13 y (525 boys and 585 girls) that was obtained using two 24-h recalls. -

Great Food Lives Here
Great food lives here. $ 99 1 ea 250G PUNNET Australian Strawberries 250g Punnet $7.96 per kg Serving suggestion $ $ 9ea 16ea SAVE $ SAVE $ UP TO 4.69 UP TO 10.50 Moccona Mainland Freeze Dried Cheese Block 1kg Coffee 400g $9.00 per kg $4.00 per 100g $ 30 $ 82 $ 4 ea 1 ea 10 ea SAVE $ SAVE $ SAVE $ UP TO 4.30 UP TO 1.83 UP TO 10.00 Connoisseur Cadbury Gourmet Favourites or Ice Cream Party Mix 570g 4-6 Pack Arnott’s Chocolate Biscuits 160-200g $1.75 per 100g On Sale 19th–25th August 2020 Novelty showbags available in selected stores. See pages 4 & 5 for more! *Showbags available at participating stores. Stocks limited. #ICHOOSESA Loved by SA since 1938 Part of the SA community since 1938, many South Australians have treasured memories of Kytons Bakery. Using the best ingredients and timeless recipes, Kytons’ multi-award winning range includes lamingtons, hot cross buns, Christmas Pudding, mince pies, cookies and more! Sharon and Darren Sutton (with Larry Lamington), owners of Kytons since 2003 at Kytons Bakery, Edwardstown. Vili Milisits in 2006 at the Vili’s Family Bakery, Mile End. An SA icon since 1968 Established in 1968, Vili’s Family Bakery is a much-loved icon across generations of South Australians. Proudly supporting local producers, Vili’s pies and cakes are made with only the freshest Australian sourced ingredients, feature our unique flaky pastry, and NO meat substitutes. We trust all of our customers will ‘Taste the Dierence’! FOODLAND P34 2020 P3 $ 5ea GREAT VALUE $ 10ea GREAT VALUE $ 30ea GREAT VALUE YELLOW BRICK ROAD #TheShowAtHome #YBRTrip Join the Yellow Brick Road Trip & SUPPORT LOCAL! RECEIVE A FREELENSWOOD APPLE When you present your #YBRTrip Map at any Foodland. -

Validation of a Checklist for Estimating Fat Intake
UNIVERSITY OF NEW SOUTH WALES Thesis/Project Report Sheet Surname or Family name: DEAKIN First name: Vicki Other name/s: Abbreviation for degree as given in the University calendar: M.Sc in Food Sc Tech School: Food Science and Technology Faculty: Applied Science Title: Validation of a Checklist for Estimating Fat Intake Abstract 350 words maximum: The objective of this study was to develop a short-term fat checklist (FC) and to examine whether it could replace a three-day food record (FR). The intended use of the FC was to measure and monitor current fat intake over three consecutive days in patients attending a local clinic in Canberra during a four-month lifestyle intervention program, and to provide an education message. A self-administered FC, in the form of a semi-quantitative FFQ, comprising 43 food items derived from food consumption data of two Australian population studies of adults was developed. The FC was compared with a three-day estimated food record (FR) in 19 volunteer clinic patients (9 men, 10 women) and also, to broaden its application, in 42 tertiary nutrition students (12 men, 30 women) against a three day weighed FR. Correlations for fat intake between the FC and FR were 0.86 for clinic patients and 0.68 for nutrition students. Mean differences in fat intake estimated by the two methods did not differ significantly from zero although Bland-Aitman plots showed large difference in fat intake between individuals. When classified by the FR, 92% of all subjects fell into the same or adjacent quartile when classified by the FC. -

Nutritional Guidelines
Science in Coaching Resource: Nutritional Guidelines Part 1: Nutritional Guidelines for Health (All Coaching Communities) Part 2: Middle/Late Childhood Coaching Communities Part 3: Early/Late Teenage Coaching Communities Part 4: Social/Competitive Adult Coaching Communitie This resource covers learning outcome 2 for the Science in Coaching Module. Part 1: Nutritional guidelines for health (All Coaching Communities) Healthy nutrition benefits any competitive athlete regardless of age, gender or level of competition. Research reveals that sprint, endurance and strength skills, for example, all benefit from the food that the athlete consumes. Good nutrition will not only benefit competition day. It will quite possibly make the athlete feel and train better and hence is an important consideration for effective day-to-day activity and training. The guidelines that follow are designed to ensure that athletes understand the correct proportions of foods to suit both training and everyday needs. Basic guidelines The points that follow meet the New Zealand guidelines for healthy eating and can be adjusted to suit an athlete’s specific sports needs. 4.1 Eat a variety of food Eating a variety of food from all the major food groups will give your body all the nutrients it requires. 6-11 serves of bread/cereal/rice/pasta – This will give carbohydrates for energy, dietary fibre and a number of vitamins and minerals 5+ a day of fruits and vegetables – This will give a boost in vitamins and minerals as well as dietary fibre and carbohydrates. 2-3 serves of meat/meat alternatives, dairy/dairy alternatives – This provides our body with protein for building and repair muscles, as well as providing a source for many vitamins (fat soluble) and minerals such as iron. -
Using Response Surface Methodology to Model the Impact of Extrusion on Starch Digestibility and Expansion of Sorghum Maize Composite Flour
School of Public Health Using response surface methodology to model the impact of extrusion on starch digestibility and expansion of sorghum maize composite flour Rebecca Lynn Licata This thesis is presented for the Degree of Master of Philosophy (Public Health) of Curtin University Nov 2012 Declaration To the best of my knowledge and belief this thesis contains no material previously published by any other person except where due acknowledgment has been made. This thesis contains no material which has been accepted for the award of any other degree or diploma in any university. ii ACKNOWLEDGEMENTS I would like to thank the following people for the help and support they gave me during this Masters thesis: The Australian Research Council (ARC) and Sanitarium Health & Wellbeing for their financial support Dr Stuart K Johnson, my supervisor, for all the time and encouragement he gave me and for not letting me quit, Thank you. Also for the support of my co- supervisors Dr Hannah Williams, Dr Ranil Coorey, and Dr Yun Zhao Jiayue (Rachel) Chu and Shilong (Tom) Wang for the great team we made running the extruder. It would have been so difficult alone and not half as much fun. Haelee Fenton, Komal Tulsidas, Imran Khan, and ChemCentre, for assistance with analyses Alan Cruickshank and the Queensland Department of Employment, Economic Development and Innovation (QDEEDI) for supply of the sorghum grain used in this study Department of Agriculture and Food Western Australia (DAFWA) for the use of the extruder and milling facilities To the Curtin University Food Science laboratory staff for their helpfulness and support whenever I was running analyses. -
Intake of Omega-3 Long Chain Polyunsaturated Fatty Acid (N-3 LCPUFA)
University of Wollongong Research Online University of Wollongong Thesis Collection University of Wollongong Thesis Collections 2013 Intake of omega-3 long chain polyunsaturated fatty acid (n-3 LCPUFA) among Australian children (2-16 years): in depth nutrient and food analysis Setyaningrum Rahmawaty University of Wollongong Recommended Citation Rahmawaty, Setyaningrum, Intake of omega-3 long chain polyunsaturated fatty acid (n-3 LCPUFA) among Australian children (2-16 years): in depth nutrient and food analysis, Doctor of Philosophy thesis, School of Health Sciences, University of Wollongong, 2013. http://ro.uow.edu.au/theses/3945 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] INTAKE OF OMEGA-3 LONG CHAIN POLYUNSATURATED FATTY ACID (n-3 LCPUFA) AMONG AUSTRALIAN CHILDREN (2-16 YEARS): In depth nutrient and food analysis A thesis submitted in fulfilment of the requirements for the award of the degree DOCTOR OF PHILOSOPHY from UNIVERSITY OF WOLLONGONG by Setyaningrum Rahmawaty Grad Dip. III Nutr (Academy of Nutrition Semarang, Indonesia) Grad Dip. IV Clin Nutr (Brawijaya University, Indonesia) MHSc. Clin Nutr (Gadjah Mada University, Indonesia) SCHOOL OF HEALTH SCIENCES 2013 Certification I, Setyaningrum Rahmawaty, declare that this thesis, submitted in partial fulfilment of the requirements for the award of Doctor of Philosophy, in the School of Health Sciences, University of Wollongong, is wholly my own work unless otherwise -

Original Article 1 Drawing on Strategic Management Approaches to Inform
1 Type: Original Article 2 Drawing on Strategic Management Approaches to Inform Nutrition Policy Design: An 3 Applied Policy Analysis for Salt Reduction in Packaged Foods 4 Abstract 5 Background: Nutrition policies to improve the food environment frequently rely on voluntary 6 business action for implementation, many have had mixed success. The aims of this study were 7 to identify key food system drivers influencing the Australian packaged food sector and analyse 8 how these might impact the willingness of food companies to voluntarily reduce salt in 9 packaged foods. 10 Methods: Business methods formed the basis of this retrospective applied policy analysis of 11 voluntary salt reduction for the period 2013-2016 where the focal policy was the Australian 12 Food and Health Dialogue (2009-2015). The analytical framework included political-legal, 13 economic, social, technological (PEST) external drivers of the food system, and Porter’s Five 14 Forces for the competitive drivers of the food system. Documentary data identifying food 15 system drivers affecting the Australian packaged food sector (comprised of the food processing 16 and supermarket industries) were identified through a comprehensive search of the grey and 17 academic literatures. 18 Results: The interplay between external and competitive food system drivers created an 19 environment in which voluntary salt reduction was found to be an uneasy fit. A high cost of 20 doing business, soft growth, intense competition, asymmetry of power in favour of 21 supermarkets, and marginal consumer interest in less salty food were found likely to create 22 commercial disincentives to invest in voluntary salt reduction above more pressing commercial 23 imperatives. -

Fathers' Impact on the Dietary and Physical Activity Behaviours of Children Aged 0‐5 Years
Fathers’ impact on the dietary and physical activity behaviours of children aged 0‐5 years Adam Walsh BAppSc (Human Movement) MNutr & Diet Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy Deakin University December, 2017 Acknowledgements There are many people who have been with me, in one way or another, throughout my PhD journey. Firstly, I must thank my supervisors without whom I couldn’t have taken the road less travelled. Karen Campbell, who would have thought a shared flight home from DAA in Darwin would end like this? Thank you for giving me the opportunity to better myself. I truly value the support you have provided throughout my journey. Your constant looking out for me has not gone unnoticed and has been greatly appreciated. Kylie Hesketh, thank you for your constant support and guidance throughout my PhD. Your insights into the nuances of research and ability to guide me with them has helped me gain the confidence I needed to believe I could actually do this. Thank you for your promotion of my work – I know this has opened doors that perhaps would have otherwise remained closed. David Crawford, I’m not sure my academic journey would be as it is without your endless support. You’ve provided guidance, advice and ceaseless encouragement in my academic life, none more so than during my PhD. The opportunities that have been afforded me are, in large part, because of you. For that I am truly obliged. Adrian Cameron, in many ways you were the sounding board of my PhD. -

The Development of a Food Frequency Questionnaire to Be Used to Identify the Dietary Intake of Phytoestrogens in a Sample Study
University of Wollongong Research Online University of Wollongong Thesis Collection 1954-2016 University of Wollongong Thesis Collections 1994 The development of a food frequency questionnaire to be used to identify the dietary intake of phytoestrogens in a sample study group of middle aged Italian women living in the Illawarra Karen Elizabeth Christian UnivFollowersity this of and Wollongong additional works at: https://ro.uow.edu.au/theses University of Wollongong Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part of this work may be reproduced by any process, nor may any other exclusive right be exercised, without the permission of the author. Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. Unless otherwise indicated, the views expressed in this thesis are those of the author and do not necessarily represent the views of the University of Wollongong. Recommended Citation Christian, Karen Elizabeth, The development of a food frequency questionnaire to be used to identify the dietary intake of phytoestrogens in a sample study group of middle aged Italian women living in the Illawarra, Master of Science thesis, Department of Public Health and Nutrition, University of Wollongong, 1994. -

Great Food Lives Here
Great food lives here. $ 29kg Australian Lamb Racks or Loin Rib Cutlets Bulk Serving suggestion $ $ 2ea 3ea SAVE $ SAVE $ UP TO 2.00 UP TO 3.00 Nice & Natural Uncle Tobys Bars 180-192g Oats 12 Pack $ $ 25 2ea 2 ea SAVE $ SAVE $ UP TO 2.00 UP TO 2.25 Campbell’s Golden Circle Real Stock Tetra Fruit Drinks 1 Litre 6 x 250ml $0.20 per 100ml $1.50 per litre $ 90 5 ea show your support SAVE $ UP TO 4.37 for our local SA almond growers Foodland Raw or Dry Roasted Almonds 400-500g FOODLAND P29 2020 P1 On Sale 15th–21st July 2020 I CHOOSE SA laura salisbury Murray Bridge Let’s all ‘Choose SA’ and support adelaide Mighty South Aussies Join us in being a Mighty South Aussie and support these GLYNDE local brands and businesses across our great state! MILE END south $ $ $ 50 5ea 1 ea 4 ea SAVE $ SAVE $ SAVE $ UP TO 2.77 UP TO 1.00 UP TO 1.00 Edwards Crossing Violet Crumble Cheese 200g Balfours Bar Cake 400-500g Medium Bars 50g $2.00 per 100g $2.25 per 100g $ 60 $ 20 $ 2 ea 3 ea 6ea SAVE $ BAKED LOCALLY SAVE $ UP TO 1.15 UP TO 2.00 Farmers Union Golden North Iced Coffee Giant Twins 600ml Vili’s Pies, Pasties 5 x 150ml $4.33 per litre or Sausage Rolls 150-165g $0.80 per 100ml FOODLAND P29 2020 P2 SUPPORTING OUR COMMUNITY 1 2 3 $ 90 Heat & eat $ 45 $ 45 10 ea 4 ea 2 ea EVERY DAY! EVERY DAY! EVERY DAY! Community Co Community Co Quiche Range Extra Virgin Community Co 700g Olive Oil 500ml Cordial 1 Litre $1.56 per 100g $0.89 per 100ml $2.45 per litre $ 50 $ 15 $ 50 Heat & eat 6 ea 4 ea 6 ea SAVE $ EVERY DAY! EVERY DAY! UP TO 2.50 Community Co Honey 500g -
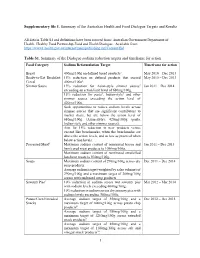
1 Supplementary File 1. Summary of the Australian Health and Food Dialogue Targets and Results Table S1. Summary of the Dialogue
Supplementary file 1. Summary of the Australian Health and Food Dialogue Targets and Results All data in Table S1 and definitions have been sourced from: Australian Government Department of Health. Healthy Food Partnership-Food and Health Dialogue. Available from: https://www1.health.gov.au/internet/main/publishing.nsf/Content/fhd. Table S1. Summary of the Dialogue sodium reduction targets and timeframe for action Food Category Sodium Reformulation Target Timeframe for action Bread 400mg/100g on defined bread products1. May 2010 – Dec 2013 Ready-to-Eat Breakfast 15% reduction on defined products that exceed May 2010 – Dec 2013 Cereal 400mg/100g2. Simmer Sauce 15% reduction for Asian-style simmer sauces3 Jan 2011 – Dec 2014 exceeding an action limit level of 680mg/100g. 15% reduction for pasta4, Indian-style5 and other simmer sauces exceeding the action level of 420mg/100g. Seek opportunities to reduce sodium levels across simmer sauces that are significant contributors to market share, but are below the action level of 680mg/100g (Asian-style); 420mg/100g (pasta, Indian-style and other simmer sauces). Aim for 15% reduction in new products versus current like benchmarks, when the benchmarks are above the action levels, and as low as practical when below action levels. Processed Meat6 Maximum sodium content of nominated bacon and Jan 2011 – Dec 2013 ham/cured meat products to 1090mg/100g. Maximum sodium content of nominated emulsified luncheon meats to 830mg/100g. Soups Maximum sodium content of 290mg/100g across dry Dec 2011 – Dec 2014 soup products. Average sodium target (weighted by sales volume) of 290mg/100g and a maximum target of 300mg/100g across wet/condensed soup products.