TSM Proficiency Testing Statistical Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
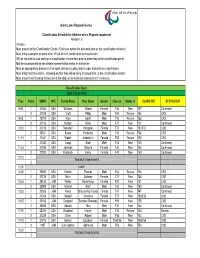
PI Classification Schedule GLRG.Xlsx
Great Lakes Regional Games Classification Schedule for Athletes with a Physical Impairment Version 1.6 Athletes - Must present to the Classification Centre 15 minutes before the allocated time on the classification schedule. Must bring a passport or some other official form of identification to classification. Will be required to read and sign a classification release form prior to presenting to the classification panel. May be accompanied by one athlete representative and/or an interpreter. Must be appropriately dressed in their sport clothes including shorts under tracksuits and sport shoes. Must bring their track chairs, strapping etc that they will be using in competition, to the classification session. Must ensure their throwing frames are at the stadium for technical assessments if necessary. Classification Day 1 Date: 9 June 2016 Time Panel SDMS NPC Family Name First Name Gender Class In Status In CLASS OUT STATUS OUT 9:00 1 31066 USA Williams Taleah Female T46 New T47 Confirmed 2 31008 USA Croft Philip Male T54 Review T54 CRS 9:45 1 15912 USA Rigo Isaiah Male T53 Review T53 CRS 2 31016 USA Nelson Brian Male F37 New F37 Confirmed 10:30 1 31218 USA Beaudoin Margaret Female T37 New T37/F37 CNS 2 30821 USA Evans Frederick Male T34 Review F34 CRS 11:15 1 11241 USA Weber Amberlynn Female T53 Review T53 CRS 2 31330 USA Langi Siale Male F43 New F43 Confirmed 11:45 1 31098 USA Johnson Shayna Female T44 New T44 Confirmed 2 27200 USA Frederick Emily Female F40 New F40 Confirmed 12:15 1 Technical Assessments 2 13:00 Lunch 14:00 1 20880 USA -

VMAA-Performance-Sta
Revised June 18, 2019 U.S. Department of Veterans Affairs (VA) Veteran Monthly Assistance Allowance for Disabled Veterans Training in Paralympic and Olympic Sports Program (VMAA) In partnership with the United States Olympic Committee and other Olympic and Paralympic entities within the United States, VA supports eligible service and non-service-connected military Veterans in their efforts to represent the USA at the Paralympic Games, Olympic Games and other international sport competitions. The VA Office of National Veterans Sports Programs & Special Events provides a monthly assistance allowance for disabled Veterans training in Paralympic sports, as well as certain disabled Veterans selected for or competing with the national Olympic Team, as authorized by 38 U.S.C. 322(d) and Section 703 of the Veterans’ Benefits Improvement Act of 2008. Through the program, VA will pay a monthly allowance to a Veteran with either a service-connected or non-service-connected disability if the Veteran meets the minimum military standards or higher (i.e. Emerging Athlete or National Team) in his or her respective Paralympic sport at a recognized competition. In addition to making the VMAA standard, an athlete must also be nationally or internationally classified by his or her respective Paralympic sport federation as eligible for Paralympic competition. VA will also pay a monthly allowance to a Veteran with a service-connected disability rated 30 percent or greater by VA who is selected for a national Olympic Team for any month in which the Veteran is competing in any event sanctioned by the National Governing Bodies of the Olympic Sport in the United State, in accordance with P.L. -

Claudia Jones Part 5 of 10
92 FEDERAL BUREAU OF INVESTIGATION CLAUDIA JONES PART 2 OF 4 FILE NUMBER : 100-72390 '_._.1-- L/400//? 92/0/ 5 / I/01,0/WE i.___ .'?lé ,1v¬ I " ' -- -1. " r=-.~ ~ A 7 32,- *-¢4 --4 , _-._' V. 1 _@~_.._ ------ ' A-7 -,.-.e_...____ $__ .-:1 ¢ -_92 ., _! "-92*,':.,=...'_,r - ti ;=w3'_ . Y:-1 e H. 5 I V , 92 . - '1 _ I ._ ,_z;~ - __ PI ''_. -- '1_1~.' .-'_-.?-. '1': gww 5"» N" T°1f1=. - _ _ October12,"i'9h9 =1 .;,> Director, FBI ', _ _ e A * J r .-=,_;~.~- +.-.1L. - - ,;_;- :~¢ , --| ' nnwmur.Cuunn sscuanr muC5cHourIc:, --c --with P. _-_-.~; teliuel =5"?9* 1;.-_;+j _"%»:'_-~.--1 M ' j. +1-.,? Your file 100-16676-; ...;.,_'.jA __ A_ L B111¢ 10°-7239'! " 1"" ~ . -= »- - . I ~ ' =.. ,= -. e- I. .'~- - --" 5- " -.- -*. 1- -K .-4'; t=.v _ ~.." "-- - e e, :. 1-. ~- - _ .a~.:_- ~= *" Q-' --= Q-' d'A_:92>>. :~ 1'1--» ll; ;_._.*-'."§"=":-=:-i4;-.1":=*,==-";"-==~-L. -- » =;* ~ - = . 92V: . ,- < _ ~_,,__I . - . -.,,' . - -= ¢'--. --3-5; ~ .__--_1 I -_; -- . * i_ '»=-E-T37:-1~ -1n An e:|:e.n:Lnationof I92I1>j0¢§-'8-fill! reects thatthe lest .i¢.-W| port submittedby youroftice is datedFeb:-uu7 L,19149,; Ly}, * "1 ' '- - 1 ~-»' =;1 -;- ..c'1 T. ate as soon as poaaiblu - - - , . , . t Please eubnit another report "HQ. -3% _, 1»-. -

Section “F” – Para Athletes Championship Competition
Section “F” – Para Athletes Championship Competition These rules are to be read in conjunction with Section ”A" Competition General Rules, Section “B” – Track Events and Section “C” – Field Events. 1. Para Athletes 1.1 General Conditions i. Athletes with a disability qualifying in any able body event at the State Track & Field Championships will not be allowed to compete in the equivalent Para event conducted on the same program. ii. Implements shall comply with the specifications as defined by LANSW for Para events. iii. In Para field events only three trials will be allowed. iv. All LANSW and IAAF (where applicable) rules of competition shall apply except in the following instances. 1.2 Classification i. Athletes with a disability have to be formerly classified by a recognised organisation, prior to competing at the State Track & Field Championships. ii. Classification is a way of grouping athletes of similar function or ability for the purpose of competition. 1.3 Competition i. All events will be conducted as multi-disability events. Competitors will compete against a multi- disability standard (MDS) as determined by the Australian Paralympic Committee. Placings will be determined by the competitor’s time/distance calculated against a percentage of the multi-disability standard (MDS). ii. In all competitions involving throwing events athletes must use the implement weight specified for their classification/ age group, (refer Para Athletes Implement Specification Table). Note: At competition events where combined age group are conducted the competitor’s will throw the weight specified for their age group. This could result in different weight implements being used in the same event. -

(FY) 2020 ICD-10-CM Official Guidelines for Coding and Reporting
ICD-10-CM Official Guidelines for Coding and Reporting FY 2020 (October 1, 2019 - September 30, 2020) Narrative changes appear in bold text Items underlined have been moved within the guidelines since the FY 2019 version Italics are used to indicate revisions to heading changes The Centers for Medicare and Medicaid Services (CMS) and the National Center for Health Statistics (NCHS), two departments within the U.S. Federal Government’s Department of Health and Human Services (DHHS) provide the following guidelines for coding and reporting using the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM). These guidelines should be used as a companion document to the official version of the ICD-10- CM as published on the NCHS website. The ICD-10-CM is a morbidity classification published by the United States for classifying diagnoses and reason for visits in all health care settings. The ICD-10-CM is based on the ICD-10, the statistical classification of disease published by the World Health Organization (WHO). These guidelines have been approved by the four organizations that make up the Cooperating Parties for the ICD-10-CM: the American Hospital Association (AHA), the American Health Information Management Association (AHIMA), CMS, and NCHS. These guidelines are a set of rules that have been developed to accompany and complement the official conventions and instructions provided within the ICD-10-CM itself. The instructions and conventions of the classification take precedence over guidelines. These guidelines are based on the coding and sequencing instructions in the Tabular List and Alphabetic Index of ICD-10-CM, but provide additional instruction. -

Petitioner's Exhibit 17
JURC PETITIONER'S EXHIBIT 0.~~.....L--- Petitioner's Exhibit 17 7 TOWN OF CEDAR LAKE Preliminary Engineering Report Cedar Lake Water Utility System Improvements July 2020 Prepared by: !,?.!! !!e.!,~JI ~ •• !.'!!; ru.E.~~ ~ Ph: (219) 844 8680 • Fax: (219) 844 7754 · e-mail: [email protected] Your Vision • Our Focus Table of Contents 1. Project Location ............................................................................................................................................ 6 2. Current Needs ............................................................................................................................................... 6 2.1. Existing System ...................................................................................................................................... 6 2.1.a. Distribution System ....................................................................................................................... 6 2.1.b. Supply System ............................................................................................................................... 7 2.1.c. Storage System ............................................................................................................................. 8 2.1.d. Treatment Systems ....................................................................................................................... 8 2.1.e. Document Needs ......................................................................................................................... -

Event Information
46th NATIONAL INCLUSIVE ATHLETICS CHAMPIONSHIPS 2018 EVENT INFORMATION 1 INTRODUCTION The Singapore Disability Sports Council (SDSC) is pleased to invite all individuals, schools, associations and clubs to participate in the National Inclusive Athletics Championships on 20 and 28 April 2018. 1.1 Objectives: ● Creating opportunities for persons with disability to compete ● Recruiting potential newcomers to the national or national development squads ● Nominating athletes to represent Singapore at major/international competitions 1.2 This Entry Pack contains: ● Event Information ● Registration Form ● Registration for Classification Form ● Protest form 2 GENERAL INFORMATION 2.1 Field (Throws) & Long Jump Events Date: 20 April 2018 (Friday) Venue: Home of Athletics. 52 Stadium Road Singapore 397724 Time: 5:00 pm (Admission). 6:00 pm (Event Start) – 10:00 pm Division: Open Division - 15 years old and above (Born in 2003 or before) Track Events Date: 28 April 2018 (Saturday) Venue: MOE (Evans Road) Stadium. 21 Evans Road Singapore 259366 Time: 5:00 pm (Admission). 6:00 pm (Event Start) – 10:00 pm Division: Open Division - 15 years old and above (Born in 2003 or before) 2.2 Eligibility: - Singaporean or PR - With either Intellectual Impairment / Visual Impairment / Cerebral Palsy / Physical Impairment / Hearing Impairment - With a valid local or international classification status - Competent in their respective events and able to meet the Minimum Qualifying Standards (MQS) (See Annex A: Events & MQS Table) 2.3 Entry Fee: $10.00 per participant (Invoice will be issued upon registration, with payment instructions.) Page 1 of 12 46th NATIONAL INCLUSIVE ATHLETICS CHAMPIONSHIPS 2018 2.4 Registration 23rd March 2018 Deadline: Email completed forms to [email protected]. -

Athletics Classification Rules and Regulations 2
IPC ATHLETICS International Paralympic Committee Athletics Classifi cation Rules and Regulations January 2016 O cial IPC Athletics Partner www.paralympic.org/athleticswww.ipc-athletics.org @IPCAthletics ParalympicSport.TV /IPCAthletics Recognition Page IPC Athletics.indd 1 11/12/2013 10:12:43 Purpose and Organisation of these Rules ................................................................................. 4 Purpose ............................................................................................................................... 4 Organisation ........................................................................................................................ 4 1 Article One - Scope and Application .................................................................................. 6 International Classification ................................................................................................... 6 Interpretation, Commencement and Amendment ................................................................. 6 2 Article Two – Classification Personnel .............................................................................. 8 Classification Personnel ....................................................................................................... 8 Classifier Competencies, Qualifications and Responsibilities ................................................ 9 3 Article Three - Classification Panels ................................................................................ 11 4 Article Four -

July 23, 6997 Washington, DC 20002 (202) 54GGGGG
InThe Mattes Of= Senate Committee an GouemmentalAflairs Special Investigation - 1996 FEC Funds Hearfag Volume Number 8 July 23, 6997 Miller Reporting Cornpan. Inc. 507 C Street, NE. Washington,DC 20002 (202) 54GGGGG FAX: (202) 5461502 Original FiIe 0723sga8.a~~251 Pages Min-Uscrlpt@Fib? ID: I564470159 Word Index included dththis Min-U-Sdm Pmml i 114 'F~dLnleica;ona.,w k-IWO ha lor rmm arc Puc '[fkhu't uhich we I haVC Nch~suspidon.hrI review IhC 1111 in~~nfliclbyrhcrcqWlhatwemr*cforimmuniimmunityuldIhe !1111 rcquesc~.the~usticc0 armrmthasnotobj~~rCdt0gnnU 1'3 response of the Justice ocprrtmcnt in opporin# lhew h '1'4 of Unmunity lor nine 02Cr pplcwho arc lnvohcd UI the 1131 requcsu. '119Hsi IaiTeemplc case and who this Codnee.therefore. can 1141 The contlict is a xrious one. pyarululy in chc [mi call upon to me. 1151 lnermth of the North adPoindexter M, kCluK I [!I9 Ycr. the four arc socomewhar lugher up in the cham 21 ita ccrUinly conclude pnsoonrlly. u I bclimmst observers pq the tcmpk, and probably that IS wi~ythey arc or more IIV do. that in the aftermath of Le North and poindcncr ,['?I interest to ~eJusticc0epucment.becaux they had a more 1w decisions. once a congnuionalcommitm muimmunity to pa1 ccndrole. therefore may be more culpable or mybe of ['ai an Individual. it effectively forecloses the posshhty of llq mreauulance. rn a rucccurul prosecution of chat kbidud re@KklQ hc In Ihe second mmcr. which few Mr.Trie. about matters about which he or she will wsufy. whch MK drhe individuals wc arc seeking immunity. -

ANNUAL REPORT 2017 1 Heading Headingcontents
ANNUAL REPORT AND FINANCIAL STATEMENTS 2017 NEW ZEALAND RIO 2016 PARALYMPIC GAMES TEAM OPENING CEREMONY PHOTO CREDIT: GETTY IMAGES PARALYMPICS NEW ZEALAND ANNUAL REPORT 2017 1 heading headingcontents 2 Officers and Officials 4 Chairman’s Report 6 Chief Executive’s Report 7 Governance Report 8 Commercial and Marketing Report 10 High Performance Report 11 High Performance Athlete Development Report 12 Community Development Report 14 Classification Report 16 Rio 2016 Paralympic Games 20 Future Paralympic Games 21 International Para Sport Results 22 Cyril Smith Legacy Fund Recipients 24 List of Paralympians 31 Financial Report 32 Directory and Statement of Compliance & Responsibility 33 Statement of Comprehensive Revenue & Expenses 34 Statement of Changes and Net Assets 35 Statement of Financial Position 36 Cash Flow Statement 37 Notes to the Accounts 45 Independent Auditor‘s Report 2 PARALYMPICS NEW ZEALAND ANNUAL REPORT 2017 officers & officials PNZ PATRON His Excellency LT GEN The Right Honourable Sir Jerry Mateparae (until August 2016) Her Excellency The Right Honourable Dame Patsy Reddy (from November 2016) PNZ BOARD Dr. Selwyn Maister QSM Ms. Catriona McBean Ms. Jana Rangooni (Chair) Mr. Mark Copeland Mr. Clive Power Ms. Jane Cotter (from February 2017) (until October 2016) Mr. Kagan Hindshaw (until Ms. Paula Tesoriero (MNZM) Mr. Duane Kale, ONZM October 2016, deceased) (from December 2016) PNZ ORDER Mr. J L McKie Mr. P Humphreys Mr. W F L Utley, OBE (deceased) OF MERIT MEMBERS Mr. J L H Savage, MBE Mr. D Kale, ONZM Mr. H J Pow (deceased) Mrs. K Condon Mr. T James Mr. P Holmes, CNZM (deceased) Mr. C Power Mr. -

U.S. Department of Veterans Affairs (VA)
U.S. Department of Veterans Affairs (VA) Veteran Monthly Assistance Allowance for Disabled Veterans Training in Paralympic and Olympic Sports Program (VMAA) In partnership with the United States Olympic Committee and other Olympic and Paralympic entities within the United States, VA supports eligible service and non-service-connected military Veterans in their efforts to represent Team USA at the Paralympic Games, Olympic Games and other international sport competitions. The VA Office of National Veterans Sports Programs & Special Events provides a monthly assistance allowance for disabled Veterans training in Paralympic sports, as well as certain disabled Veterans selected for or competing with the national Olympic Team, as authorized by 38 U.S.C. 322(d) and Section 703 of the Veterans’ Benefits Improvement Act of 2008. Through the program, VA will pay a monthly allowance to a Veteran with either a service-connected or non-service-connected disability if the Veteran meets the minimum military standards or higher (i.e. Emerging Athlete or National Team) in his or her respective Paralympic sport at a recognized competition. In addition to making the VMAA standard, an athlete must also be nationally or internationally classified by his or her respective Paralympic sport federation as eligible for Paralympic competition. VA will also pay a monthly allowance to a Veteran with a service-connected disability rated 30 percent or greater by VA who is selected for a national Olympic Team for any month in which the Veteran is competing in any event sanctioned by the National Governing Bodies of the Olympic Sport in the United State, in accordance with P.L. -

ANNUAL REPORT Financial Statements
ANNUAL REPORT Financial Statements 2005 – 2006 Torino 2006 Paralympic Games Contents Officers and Officials …………..3 Chairman’s Report …………..4 Chief Executive Report …………..4 PNZ Board .…………..5 PNZ Staff & Service Providers .…………..6 SPARC .…………..7 International Paralympic Committee .…………..7 Sponsors & Supporters .…………..8 High Performance Report ………….9 Lion Foundation Paralympic Academy .………….9 Sports Science Report .………….10 Athletes Report ………….10 International Teams and Results ………….12 PA/NZAS Carded Athletes ………….19 PNZ / NZAS Sport Liaison .………….19 Operations Report ………… 19 Key Relationships ……….….19 Events ……….….20 PNZ National Championships ……….….20 Beijing 2008 Planning / IT .………….20 Classification Report ………….21 Classifiers ….……….23 New Year Honours ..………..24 Obituary’s ..………..24 Financial Statements …………..F Statement of Financial Performance .……….…F1 Statement of Financial Position .……….…F2 Notes to the Accounts .……….…F3 Auditors Report ……….….F7 New Zealand Paralympians …………25 Strategic Plan 05-09 …………28 Sponsors and Partners …………29 2 Officers and Officials Patron Mr. Paul Holmes, NZOM Board Mr. Simon Peterson (Chair) Ms. Sandra Blewett, MBE Mr. Ross Darrah Mr. Marc Frewin (co-opted June 06) Mrs. Gillian Hall Mr. Duane Kale Mr. David Rutherford Athletes Representative Mr. Tim Prendergast & Mr. Matt Slade Honorary Solicitor Mr. John Wiltshire, LLB Auditors Hayes Knight & Co Bankers ASB Bank Ltd, Remuera, Auckland Support Office Staff Chief Executive Officer Mr. Craig Hobbs High Performance Manager Ms. Helen Murphy Operations Manager Mr. Vaughan Cruickshank (to Feb 06) Ms. Fiona Allan (from May 06) Administration Mrs. Val Hall Operations Officer Mr. Wade Chang Medical Director Dr. Paul Wharam, BM, DRCOG, FRNZGP, Dip Sports Med. Sports Science Coordinator University of Canterbury; Mr Malcolm Humm Classification Coordinator Mrs. Rebecca Foulsham (to June 06) Mrs.