CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Payment Locations - Muthoot
Payment Locations - Muthoot District Region Br.Code Branch Name Branch Address Branch Town Name Postel Code Branch Contact Number Royale Arcade Building, Kochalummoodu, ALLEPPEY KOZHENCHERY 4365 Kochalummoodu Mavelikkara 690570 +91-479-2358277 Kallimel P.O, Mavelikkara, Alappuzha District S. Devi building, kizhakkenada, puliyoor p.o, ALLEPPEY THIRUVALLA 4180 PULIYOOR chenganur, alappuzha dist, pin – 689510, CHENGANUR 689510 0479-2464433 kerala Kizhakkethalekal Building, Opp.Malankkara CHENGANNUR - ALLEPPEY THIRUVALLA 3777 Catholic Church, Mc Road,Chengannur, CHENGANNUR - HOSPITAL ROAD 689121 0479-2457077 HOSPITAL ROAD Alleppey Dist, Pin Code - 689121 Muthoot Finance Ltd, Akeril Puthenparambil ALLEPPEY THIRUVALLA 2672 MELPADAM MELPADAM 689627 479-2318545 Building ;Melpadam;Pincode- 689627 Kochumadam Building,Near Ksrtc Bus Stand, ALLEPPEY THIRUVALLA 2219 MAVELIKARA KSRTC MAVELIKARA KSRTC 689101 0469-2342656 Mavelikara-6890101 Thattarethu Buldg,Karakkad P.O,Chengannur, ALLEPPEY THIRUVALLA 1837 KARAKKAD KARAKKAD 689504 0479-2422687 Pin-689504 Kalluvilayil Bulg, Ennakkad P.O Alleppy,Pin- ALLEPPEY THIRUVALLA 1481 ENNAKKAD ENNAKKAD 689624 0479-2466886 689624 Himagiri Complex,Kallumala,Thekke Junction, ALLEPPEY THIRUVALLA 1228 KALLUMALA KALLUMALA 690101 0479-2344449 Mavelikkara-690101 CHERUKOLE Anugraha Complex, Near Subhananda ALLEPPEY THIRUVALLA 846 CHERUKOLE MAVELIKARA 690104 04793295897 MAVELIKARA Ashramam, Cherukole,Mavelikara, 690104 Oondamparampil O V Chacko Memorial ALLEPPEY THIRUVALLA 668 THIRUVANVANDOOR THIRUVANVANDOOR 689109 0479-2429349 -

Scheduled Caste Sub Plan (Scsp) 2014-15
Government of Kerala SCHEDULED CASTE SUB PLAN (SCSP) 2014-15 M iiF P A DC D14980 Directorate of Scheduled Caste Development Department Thiruvananthapuram April 2014 Planng^ , noD- documentation CONTENTS Page No; 1 Preface 3 2 Introduction 4 3 Budget Estimates 2014-15 5 4 Schemes of Scheduled Caste Development Department 10 5 Schemes implementing through Public Works Department 17 6 Schemes implementing through Local Bodies 18 . 7 Schemes implementing through Rural Development 19 Department 8 Special Central Assistance to Scheduled C ^te Sub Plan 20 9 100% Centrally Sponsored Schemes 21 10 50% Centrally Sponsored Schemes 24 11 Budget Speech 2014-15 26 12 Governor’s Address 2014-15 27 13 SCP Allocation to Local Bodies - District-wise 28 14 Thiruvananthapuram 29 15 Kollam 31 16 Pathanamthitta 33 17 Alappuzha 35 18 Kottayam 37 19 Idukki 39 20 Emakulam 41 21 Thrissur 44 22 Palakkad 47 23 Malappuram 50 24 Kozhikode 53 25 Wayanad 55 24 Kaimur 56 25 Kasaragod 58 26 Scheduled Caste Development Directorate 60 27 District SC development Offices 61 PREFACE The Planning Commission had approved the State Plan of Kerala for an outlay of Rs. 20,000.00 Crore for the year 2014-15. From the total State Plan, an outlay of Rs 1962.00 Crore has been earmarked for Scheduled Caste Sub Plan (SCSP), which is in proportion to the percentage of Scheduled Castes to the total population of the State. As we all know, the Scheduled Caste Sub Plan (SCSP) is aimed at (a) Economic development through beneficiary oriented programs for raising their income and creating assets; (b) Schemes for infrastructure development through provision of drinking water supply, link roads, house-sites, housing etc. -
District Functionaries
DISTRICT FUNCTIONARIES Kollam District DESIGNATION OFFICE PHONE/FAX MOBILE E-MAIL ID DISTRICT COLLECTOR 0474 2794900 9447795500 [email protected] DISTRICT POLICE CHIEF, KOLLAM 0474 2764422 9497996984 [email protected] CITY DISTRICT POLICE CHIEF, KOLLAM 0474 2450168 9497996908 [email protected] RURAL DY. COLLECTOR (ELECTION) 0474 2798290 8547610029 JS (ELECTION) 9496409857 [email protected] 0474 2796675 ELECTION ASSISTANT 9846110055 CORPORATION NO & NAME OF LB RO, ERO, SEC DESIGNATION OFFICE No. MOBILE E-MAIL ID RO (Wards 01 - 28) Deputy Director, Economics & 0474 2793418 9495439709 [email protected] Statistics, Kollam Assistant Conservator of Forests RO (Wards 01 - 28) 0474 2748976 9447979132 [email protected] (Social Forestry), Kollam C 02 KOLLAM CORPORATION ERO Additional Secretary, Kollam 0474 2749860 9447964511 Corporation [email protected] SECRETARY Secretary, Kollam Corporation 0474 2742724 9447413433 MUNICIPALITIES RO, ERO & OFFICE NO & NAME OF LB DESIGNATION MOBILE E-MAIL ID Secretary PHONE/FAX District Soil Conservation Officer, RO 0474 2768816 9447632532 [email protected] Kollam M 05 Paravur Municipality ERO Secretary, Paravur Municipality 0474 2512340 8281286929 [email protected] Divisional Forest Officer, Timbersales RO 0475 2222617 9847021389 [email protected] M 06 Punalur Municipality Division, Punalur ERO Secretary, Punalur Municipality 0475 2222683 9037568221 [email protected] Joint Director of Co operative Audit, RO 0474 2794923 9048791068 jdaklm@co_op.kerala.gov.in Kollam -

AMRITA SCHOOL of AYURVEDA DEPARTMENT of POST GRADUATE STUDIES LIST of SYNOPSIS, GUIDE & CO-GUIDE Department of Rashashastra
AMRITA SCHOOL OF AYURVEDA DEPARTMENT OF POST GRADUATE STUDIES LIST OF SYNOPSIS, GUIDE & CO-GUIDE Department of Rashashastra & Bhaisajya Kalpana Roll No Scholar Title Of Synopsis Guide Co-Guide A Pharmaceutico - Clinical Study Of Krimimudgara Rasa In Dr. Abhaya Kumar 13. Dr Chitra M.S. Dr. K. Unnikrishnan Pillai Udarakrimi Mishra “A Comparative Pharmaceutico-Analytical Study Of 14. Dr. Divya Ravindran Balarishta Prepared With Dhataki Pushpa And Yeast As Dr.Abhaya Kumar Mishra, Dr. Arun Mohan Sandhana Dravyas” “Physico-Chemical Analysis Of Kasisa Purified By Different 15. Dr. Pooja P Bhavana Dravyas And Their Effect In Haemoglobin Level - A Dr.K.Unnikrishna Pillai. Dr. Ramesh.N.V., Comparative Study.” “Pharmaco-Analytical Study And In Vitro Antibacterial 16. Dr. Remya.A. Effect Of Swasananda Gulika In Selected Respiratory Dr.Abhaya Kumar Mishra, Dr. Arun Mohanan Pathogens” Comparative Physico-Chemical Analysis Of Ksheerabala 17. Dr. Prajeesh Nath Dr. Ramesh N. V. Dr. Arun Mohanan Taila W.S.R To Avartana (Fortification) “A Pharmaceutico-Analytical Study Of Pravala Pishti And Its Dr. Abhaya Kumar 18. Dr. Priya Raghunathan Clinical Efficacy On Hypocalcaemia In Menopausal Dr.K.Unnikrishna Pillai., Mishra Women.” AMRITA SCHOOL OF AYURVEDA AMRITA VISHWA VIDYAPEETHAM (University under sec.3 UGC Act 1956) PROFORMA FOR REGISTRATION OF SUBJECT FOR DISSERTATION FOR AYURVEDA VACHASPATI M.D (AYU) IN RASA SHASTRA AND BHAISHAJYA KALPANA A PHARMACEUTICO - CLINICAL STUDY OF KRIMIMUDGARA RASA IN UDARAKRIMI BY Dr. CHITHRA M.S (Ist YEAR P.G. SCHOLAR) DEPT. OF P.G STUDIES IN RASA SHASTRA AND BHAISHAJYA KALPANA AMRITA SCHOOL OF AYURVEDA, VALLIKAVU, CLAPPANA POST, KOLLAM GUIDE DR.K.UNNIKRISHNA PILLAI., M.D (Ayu.), Ph.D PROFESSOR AND H.O.D. -

Annexure 1 B - Kollam
Annexure 1 B - Kollam Allotted Mobile Nos Sl.No Designation/Post Allotted Office District Allotted 1 Kollam 9383470770 PAO Kollam District Office Kollam 2 Kollam 9383470102 JDA PDATMA KLM ATMA KLM 3 Kollam 9383470208 AO KB Nedumpana Chathannoor Block 4 Kollam 9383470210 AO KB Kalluvathukkal Chathannoor Block 5 Kollam 9383470213 AO KB Chirakkara Chathannoor Block 6 Kollam 9383470215 AO KB Chathannoor Chathannoor Block 7 Kollam 9383470217 AO KB Adichanelloor Chathannoor Block 8 Kollam 9383470219 AO KB Poothakulam Chathannoor Block 9 Kollam 9383470224 AO KB Paravoor Chathannoor Block 10 Kollam 9383470225 AO KB Sasthamkotta Sasthamcotta Block 11 Kollam 9383470227 AO KB Kunnathur Sasthamcotta Block 12 Kollam 9383470229 AO KB Poruvazhy Sasthamcotta Block 13 Kollam 9383470231 AO KB Sooranadu North Sasthamcotta Block 14 Kollam 9383470233 AO KB Sooranadu South Sasthamcotta Block 15 Kollam 9383470236 AO KB Mynagapally Sasthamcotta Block 16 Kollam 9383470238 AO KB West Kallada Sasthamcotta Block 17 Kollam 9383470316 DD(WM) PAO KLM 18 Kollam 9383470317 DD (NWDPRA) PAO KLM 19 Kollam 9383470318 DD (C ) PAO KLM 20 Kollam 9383470319 DD (YP) PAO KLM 21 Kollam 9383470320 DD (E &T) PAO KLM 22 Kollam 9383470313 DD (H) PAO KLM 23 Kollam 9383470230 TA PAO KLM 24 Kollam 9383470330 APAO PAO KLM 25 Kollam 9383470240 ACO PAO KLM 26 Kollam 9383470347 AA PAO KLM 27 Kollam 9383470550 ADA (Marketing) PAO KLM 28 Kollam 9383470348 ASC DSTL KLM 29 Kollam 9383470338 AO DSTL KLM 30 Kollam 9383470339 ASC MSTL KLM 31 Kollam 9383470331 AO MSTL KLM 32 Kollam 9383470332 ADA -

Kollam School Code Sub District Name of School School Type 41001 Chathannoor Govt
Kollam School Code Sub District Name of School School Type 41001 Chathannoor Govt. H S S Bhoothakulam G 41002 Chathannoor Chempakassery H S S A 41003 Chathannoor N S S H S S Chathannoor A 41004 Chathannoor Nehru Memorial HSS U 41005 Chathannoor Adichanalloor Panchayat H S G 41006 Chathannoor Govt. H S Chathannoor G 41007 Chathannoor Govt. H S Nedungolam G 41008 Chathannoor Govt. H S Uliyanad G 41009 Chathannoor Kalluvathukkal Panchayat H S G 41010 Chathannoor Amirita Sanskrit H S S A 41011 Chathannoor Ezhippuram H S S A 41012 Chavara Govt. H S S Chavara G 41013 Chavara Lourde Matha English Medium H S, Kovilthottam U 41014 Chavara Govt. H S for Girls Chavara G 41015 Chavara Govt. H.S.S Panmanamanayil G 41016 Chavara Guhanandapuram H S S Chavara South A 41017 Karunagappally Govt. V H S S Cheriazheekal G 41018 Karunagappally Govt. R F T H S Karunagappally G 41019 Karunagappally S V H S S Clappana A 41020 Karunagappally Govt. Fishery H S S Kuzhithura G 41021 Kundara K P S P M V H S S East Kallada A 41022 Kundara St. Margarets G H S Kanjirakode A 41023 Kundara Sivaram N S S H S S Karicode A 41024 Kollam MEAM English Medium H S S U 41025 Kundara C V K M H S S East Kallada A 41026 Kundara M M H S Uppoodu A 41027 Kundara R S M H S Pazhangalam A 41028 Kundara Govt. H S Keralapuram G 41029 Kollam Govt. H S S Mangad G 41030 Kollam Govt. -

Final Report
1 REPORT ON CRZ VIOLATIONS IN KOLLAM DISTRICT 1. INTRODUCTION As per the direction of Supreme Court to prepare the list of violations against CRZ Notification across the State, Government of Kerala vide Order No G.O (Rt) No 98/2017/Envt. dated Thiruvananthapuram, 16/10/2019 (Annexure 1) have constituted Coastal District Committees (CDC) for ten coastal districts including the district of Kollam with District Collector as Chairman and District Town Planner as Convener for preparing the list of violations against CRZ Notification. All the concerned local body secretaries and Village officers are the members. Accordingly, the first meeting of CDC, Kollam was convened on 24.10.2019 and an Action Plan for collection and compilation of list of CRZ violations was discussed and decided. (Minutes of first meeting is enclosed as Annexure II.) As neither guidelines nor prescribed formats for the collection of list of violations against CRZ Notification were provided, it is decided in the first CDC meeting to collect the details in two phases. In the first phase, the focus was to collect location wise (i.e., survey number wise) number of CRZ violations in every village included in CRZ Notification. Category of violations such as residential, commercial etc. and land development were also to be identified. In the second phase details such as name and address of owners, status of owners, distance to the violations from HTL, area of construction/land etc. of identified CRZ violations were to be collected. Accordingly, Ist,, IInd and IIIrd Interim Report on CRZ violations were prepared based on the formats (Annexure III) issued to all concerned local bodies and the reports were submitted to the Chief Secretary to Government on due dates viz.31-10-19, 30-11-19 and 20-12-19 respectively. -

Accused Persons Arrested in Kollam City District from 21.06.2020To27.06.2020
Accused Persons arrested in Kollam City district from 21.06.2020to27.06.2020 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Address of Cr. No & Sec Police father of which Time of Officer, which No. Accused Sex Accused of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 1 2 3 4 5 6 7 8 9 10 11 Cr.2120/2020 U/S 269, 188, SHEMEERA 270 IPC & MANZIL, 4(2)(a) r/w 5 MUHAMMED Male, 1 SABU PEOPELES NAGAR Kadappakkada 21.06.2020 of Kerala Kollam East SI of Police Station Bail HANEEFA Age:37 337, Epidemic KADAPPAKKADA Disease Ordinance 2020 Cr.2121/2020 U/S 269, 188, ANUGRAHA 270 IPC & NAGAR 190, 4(2)(a) r/w 5 Male, 2 JOSE VARGEESE PALLITHOTTAM, Kadappakkada 21.06.2020 of Kerala Kollam East SI of Police Station Bail Age:27 KOLLAM EAST Epidemic Police Station Disease Ordinance 2020 Cr.2122/2020 U/S 269, 188, 270 IPC & BHDRADEEPAM, 4(2)(a) r/w 5 Male, 3 GLEN MARY DALE, Kadappakkada 21.06.2020 of Kerala Kollam East SI of Police Station Bail CHRISTPHER Age:32 NrVANCHKOVIL Epidemic Disease Ordinance 2020 Cr.2123/2020 U/S 269, 188, PEROOR 270 IPC & VADAKKATHIL, 4(2)(a) r/w 5 Male, 4 SHEFEEK SHARAFUDE VALANTHUNGAL, Pulimoodu 21.06.2020 of Kerala Kollam East SI of Police Station Bail Age:31 EN ERAVIPURAM Epidemic Police Station Disease Ordinance 2020 Cr.2125/2020 U/S 269, 188, PUTHUVAL 270 IPC & PURAYIDOM, 4(2)(a) r/w 5 Male, 5 NISHAD RAJU BEECH NAGAR58 Mundakkal 21.06.2020 of Kerala Kollam East SI of Police Station Bail Age:20 MUNDAKKAL, Epidemic KOLLAM Disease Ordinance -

Camps in Kollam District
Camps in Kollam District 1. Type A camps - General Public 2. Type B camps- Elderly people 3. Type C camps - People with COVID-19 Symptoms 4. Type D camps- People in quarantine Type of Accessible Sl. No Name of the Building LSGI Village Latitude Longitude Camp Building 1 Marthomaschool Kottarakkara Municipality Kottarakkara 9.005851 76.778095 Type A Yes - ഉണ്ട് 2 Navodaya Kottarakkara Municipality Kottarakkara 8.984338 76.764857 Type A Yes - ഉണ്ട് Karunagappally Ayanivelikulanga 3 Karunagappally Municipality 9 76 Type A Yes - Municipality ra ഉണ്ട് Boys higher secondary 4 Kottarakkara Municipality Kottarakkara 9.000353 76.774148 Type A Yes - school ഉണ്ട് 5 Govt Model Boys H.S.S Kollam Corporation Kollam West 8.894647 76.5779009 Type A Yes - ഉണ്ട് Parippally Panchayath Kalluvathukkal Grama 6 Parippally 8.811043 76.761009 Type A Yes - Community Hall Panchayat ഉണ്ട് Thrikkovilvattom Grama 7 G.L.P.S. Mukhathala Thrikkovilvattom 8.8929181 76.6496564 Type A Yes - Panchayat ഉണ്ട് West Kallada Grama 8 S.N Central School West Kallada 9.011 76.601 Type A Yes - Panchayat ഉണ്ട് Adichanalloor Grama 9 Mylakkad ups Adichanallur 8.864393 76.689962 Type A Yes - Panchayat ഉണ്ട് Pattazhi Vadakkekara Pattazhy 10 G U P S Earathuvadakku 9.1 76.79 Type A Yes - Grama Panchayat Vadakkekkara ഉണ്ട് Alayamon Grama 11 Govt H.S.S Karukone Alayaman 8.903864 76.935231 Type A Yes - Panchayat ഉണ്ട് Karavaloor Grama 12 Govt L.P.S, Karavaloor Karavaloor 8.98271 76.924639 Type A Yes - Panchayat ഉണ്ട് Thekkumbhagom Grama Thekkumbhaga 13 Govt ups thekkumbhagom 8 76 Type A Yes -

Members of the Local Authorities Kollam District
Price. Rs. 150/- per copy UNIVERSITY OF KERALA Election to the Senate by the member of the Local Authorities- (Under Section 17-Elected Members (7) of the Kerala University Act 1974) Electoral Roll of the Members of the Local Authorities- Kollam District Roll Name of Members Name of local Authorities Address No. MEMBER, ADICHANALLOOR SARASAMANI SOUHRIDAM, THAZHUTHALA, 691571 1 GRAMA PANCHAYAT MEMBER, ADICHANALLOOR NANDANAM, VADAKKE MYLAKKAD, BIJI RAJENDRAN 2 GRAMA PANCHAYAT KANNANALLOOR, 691576 MEMBER, ADICHANALLOOR SANTHOSH BHAVAN, NORTH MYLAKKAD, ROYSON 3 GRAMA PANCHAYAT KANNANALLOOR 691576 MEMBER, ADICHANALLOOR SURYA BHAVAN, PLAKKAD , M.SUBHASH 4 GRAMA PANCHAYAT ADICHANALLOOR P.O,691573 MEMBER, ADICHANALLOOR PALAVILA VEEDU, ADICHANALLOOR , AMRITHA. S 5 GRAMA PANCHAYAT ADICHANALLOOR P. O,691573 MEMBER, ADICHANALLOOR NEDIYAZHIKAM, KUNDUMON , K. NAZARUDEEN 6 GRAMA PANCHAYAT ADICHANALLOOR P. O MEMBER, ADICHANALLOOR SUBU BHAVAN , VELICHIKKALA P. O, OMANA BABU 7 GRAMA PANCHAYAT VELICHIKKALA MEMBER, ADICHANALLOOR MULAMOOTTIL , VELICHIKKALA, THOMAS JECOB 8 GRAMA PANCHAYAT VELICHIKKALA MEMBER, ADICHANALLOOR JOSHVA BHAVAN , KUMMALLOOR, J.L SHEEJA 9 GRAMA PANCHAYAT KUMMALLOOR P. O,691573 MEMBER, ADICHANALLOOR S. S. BHAVAN , KUMMALLOOR, SULOCHANA.S 10 GRAMA PANCHAYAT KUMMALLOOR P. O, 691573 MEMBER, ADICHANALLOOR MADHU MANDHIRAM, KAITHAKUZHY, MADHUSOODHANAN 11 GRAMA PANCHAYAT ADICHANALLOOR P. O, 691573 MEMBER, ADICHANALLOOR MUNNAM VADAKKATHIL VEEDU, N. AJAYAKUMAR 12 GRAMA PANCHAYAT MYLAKKAD, MYLAKKAD P. O, 691571 MEMBER, ADICHANALLOOR NETTOOR KUNNATHU VEEDU, MYLAKKAD , RAMLA BASHEER 13 GRAMA PANCHAYAT MYLAKKAD P. O , 691571 MEMBER, ADICHANALLOOR PLAVILA VEEDU, KOTTIYAM , KOTTIYAM P. O REKHA. S. CHANDRAN 14 GRAMA PANCHAYAT 691571 MEMBER, ADICHANALLOOR SHAJU NIVAS, KOTTIYAM , KOTTIYAM P. O, NADEERA KOCHASSAN 15 GRAMA PANCHAYAT 691571 MEMBER, ADICHANALLOOR HEMALAYAM , VADAKKE MYLAKKAD, HEMA SATHEESH 16 GRAMA PANCHAYAT KANNANALLOOR P. -

CTRI Trial Data
PDF of Trial CTRI Website URL - http://ctri.nic.in Clinical Trial Details (PDF Generation Date :- Tue, 28 Sep 2021 14:53:09 GMT) CTRI Number CTRI/2017/03/008048 [Registered on: 08/03/2017] - Trial Registered Prospectively Last Modified On 22/11/2017 Post Graduate Thesis No Type of Trial Interventional Type of Study Ayurveda Study Design Single Arm Trial Public Title of Study A test is done with sweta gunja (Abrus precatorius) application in nasal polyp to assess the before and after outcome. Scientific Title of A pre and post test clinical study to assess the treatment outcomes of Sweta- Gunja Lepa (Abrus Study Precatorius Application) in Ethmoidal Nasal Polyp Secondary IDs if Any Secondary ID Identifier NIL NIL Details of Principal Details of Principal Investigator Investigator or overall Name ksivabalaji Trial Coordinator (multi-center study) Designation assistant Professor Affiliation amrita school of ayurveda Address Amrita school Of Ayurveda , vallikavu, Clappana Post, kollam , kerala Amrita school Of Ayurveda , vallikavu, Clappana Post, kollam , kerala Kollam KERALA 690525 India Phone 7559027947 Fax Email [email protected] Details Contact Details Contact Person (Scientific Query) Person (Scientific Name ksivabalaji Query) Designation assistant Professor Affiliation amrita school of ayurveda Address Amrita school Of Ayurveda , vallikavu, Clappana Post, kollam , kerala Amrita school Of Ayurveda , vallikavu, Clappana Post, kollam , kerala Kollam KERALA 690525 India Phone 7559027947 Fax Email [email protected] Details Contact -
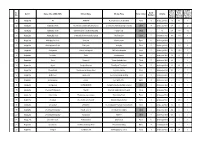
Sl. No. District Name of the LSGD (CDS)
LUNCH LUNCH LUNCH Sl. No Of Parcel Home Sponsored District Name of the LSGD (CDS) Kitchen Name Kitchen Place Rural / Urban Initiative No. Members By Unit Delivery by LSGI's (April 7) (April 7) (April 7) 1 Alappuzha Ala JANATHA Near CSI church, Kodukulanji Rural 5 Janakeeya Hotel 27 40 0 2 Alappuzha Alappuzha North Ruchikoottu Janakiya Bhakshanasala Coir Machine Manufacturing Company Urban 4 Janakeeya Hotel 107 0 20 3 Alappuzha Alappuzha South Samrudhi janakeeya bhakshanashal Pazhaveedu Urban 5 10 256 171 12 4 Alappuzha Alappuzha South Community kitchen thavakkal group MCH junction Urban 5 Janakeeya Hotel 96 144 0 5 Alappuzha Ambalppuzha North Swaruma Neerkkunnam Rural 10 Janakeeya Hotel 0 0 0 6 Alappuzha Ambalappuzha South Patheyam Amayida Rural 5 Janakeeya Hotel 0 229 6 7 Alappuzha Arattupuzha Hanna catering unit JMS hall,arattupuzha Rural 6 Janakeeya Hotel 30 135 0 8 Alappuzha Arookutty Ruchi Kombanamuri Rural 5 Janakeeya Hotel 79 57 0 9 Alappuzha Aroor Navaruchi Vyasa charitable trust Rural 5 Janakeeya Hotel 38 0 0 10 Alappuzha Aryad Anagha Catering Near Aryad Panchayat Rural 5 Janakeeya Hotel 80 60 0 11 Alappuzha Bharanikavu Sasneham Janakeeya Hotel Koyickal chantha Rural 5 Janakeeya Hotel 182 0 0 12 Alappuzha Budhanoor sampoorna mooshari parampil building Rural 5 Janakeeya Hotel 0 0 0 13 Alappuzha Chambakulam Jyothis Near party office Rural 4 Janakeeya Hotel 0 0 0 14 Alappuzha Chenganoor SRAMADANAM chengannur market building complex Urban 5 Janakeeya Hotel 30 35 0 15 Alappuzha Chennam Pallippuram Friends Chennam pallipuram panchayath