Coccolithophores and Calcite Saturation State in the Baltic and Black Seas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Overcalcified Forms of the Coccolithophore Emiliania Huxleyi in High CO2 Waters Are Not Pre-Adapted to Ocean Acidification
Biogeosciences Discuss., https://doi.org/10.5194/bg-2017-303 Manuscript under review for journal Biogeosciences Discussion started: 6 September 2017 c Author(s) 2017. CC BY 4.0 License. Overcalcified forms of the coccolithophore Emiliania huxleyi in high CO2 waters are not pre-adapted to ocean acidification. Peter von Dassow1,2,3*, Francisco Díaz-Rosas1,2, El Mahdi Bendif4, Juan-Diego Gaitán-Espitia5, Daniella Mella-Flores1, Sebastian Rokitta6, Uwe John6, and Rodrigo Torres7,8 5 1 Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile. 2 Instituto Milenio de Oceanografía de Chile. 3 UMI 3614, Evolutionary Biology and Ecology of Algae, CNRS-UPMC Sorbonne Universités, PUCCh, UACH. 4 Department of Plant Sciences, University of Oxford, OX1 3RB Oxford, UK. 5 CSIRO Oceans and Atmosphere, GPO Box 1538, Hobart 7001, TAS, Australia. 10 6 Marine Biogeosciences | PhytoChange Alfred Wegener Institute – Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany. 7 Centro de Investigación en Ecosistemas de la Patagonia (CIEP), Coyhaique, Chile. 8 Centro de Investigación: Dinámica de Ecosistemas marinos de Altas Latitudes (IDEAL), Punta Arenas, Chile. Correspondence to: Peter von Dassow ([email protected]) 15 Abstract. Marine multicellular organisms inhabiting waters with natural high fluctuations in pH appear more tolerant to acidification than conspecifics occurring in nearby stable waters, suggesting that environments of fluctuating pH hold genetic reservoirs for adaptation of key groups to ocean acidification (OA). The abundant and cosmopolitan calcifying phytoplankton Emiliania huxleyi exhibits a range of morphotypes with varying degrees of coccolith mineralization. We show that E. huxleyi populations in the naturally acidified upwelling waters of the Eastern South Pacific, where pH drops below 20 7.8 as is predicted for the global surface ocean by the year 2100, are dominated by exceptionally overcalcified morphotypes whose distal coccolith shield can be almost solid calcite. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

Bothnian Bay Coastal Meadows Management Project
EUROPEAN LANDSCAPE CONVENTION LANDSCAPE AWARD OF THE COUNCIL OF EUROPE 7th Session – 2020-2021 APPLICATION FORM Council of Europe – European Landscape Convention Presentation The European Landscape Convention aims to promote the protection, management and planning of landscapes and to bring together European co-operation in this field. It is the first international treaty exclusively devoted to all dimensions of European landscape. Taking into account the landscape, natural and cultural values of the territory, it contributes to promoting the quality of life and well-being of Europeans. The Resolution on the Rules governing the Landscape Award of the Council of Europe, adopted by the Committee of Ministers on 20 February 2008 at the 1018th meeting of the Ministers’ Deputies, draws attention to the fact that Article 11 of the Convention institutes the Landscape Award of the Council of Europe and that it is in keeping with the work carried out by the Council of Europe concerning human rights, democracy and sustainable development. It effectively promotes the territorial dimension of human rights and democracy by acknowledging the importance of measures taken to improve the landscape for people’s living conditions. Opened to the Parties to the Convention, the Award is intended to raise civil society’s awareness of the value of landscapes, of their role and of changes to them. Its objective is to reward exemplary practical initiatives aimed at successful landscape quality objectives on the territories of the Parties to the Convention. The Award is conferred every two years and the files presenting applications must reach the Secretariat General of the Council of Europe. -
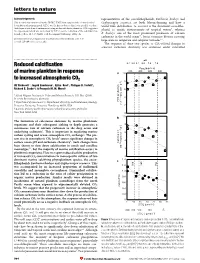
Reduced Calcification of Marine Plankton in Response to Increased
letters to nature Acknowledgements representatives of the coccolithophorids, Emiliania huxleyi and This research was sponsored by the EPSRC. T.W.F. ®rst suggested the electrochemical Gephyrocapsa oceanica, are both bloom-forming and have a deoxidation of titanium metal. G.Z.C. was the ®rst to observe that it was possible to reduce world-wide distribution. G. oceanica is the dominant coccolitho- thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested phorid in neritic environments of tropical waters9, whereas the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a. E. huxleyi, one of the most prominent producers of calcium carbonate in the world ocean10, forms extensive blooms covering Correspondence and requests for materials should be addressed to D. J. F. large areas in temperate and subpolar latitudes9,11. (e-mail: [email protected]). The response of these two species to CO2-related changes in seawater carbonate chemistry was examined under controlled ................................................................. pH Reduced calci®cation 8.4 8.2 8.1 8.0 7.9 7.8 PCO2 (p.p.m.v.) of marine plankton in response 200 400 600 800 a 10 to increased atmospheric CO2 ) 8 –1 Ulf Riebesell *, Ingrid Zondervan*, BjoÈrn Rost*, Philippe D. Tortell², d –1 Richard E. Zeebe*³ & FrancËois M. M. Morel² 6 * Alfred Wegener Institute for Polar and Marine Research, P.O. Box 120161, 4 D-27515 Bremerhaven, Germany mol C cell –13 ² Department of Geosciences & Department of Ecology and Evolutionary Biology, POC production Princeton University, Princeton, New Jersey 08544, USA (10 2 ³ Lamont-Doherty Earth Observatory, Columbia University, Palisades, New York 10964, USA 0 ............................................................................................................................................. -

Changing Communities of Baltic Coastal Fish Executive Summary: Assessment of Coastal fi Sh in the Baltic Sea
Baltic Sea Environment Proceedings No. 103 B Changing Communities of Baltic Coastal Fish Executive summary: Assessment of coastal fi sh in the Baltic Sea Helsinki Commission Baltic Marine Environment Protection Commission Baltic Sea Environment Proceedings No. 103 B Changing Communities of Baltic Coastal Fish Executive summary: Assessment of coastal fi sh in the Baltic Sea Helsinki Commission Baltic Marine Environment Protection Commission Editor: Janet Pawlak Authors: Kaj Ådjers (Co-ordination Organ for Baltic Reference Areas) Jan Andersson (Swedish Board of Fisheries) Magnus Appelberg (Swedish Board of Fisheries) Redik Eschbaum (Estonian Marine Institute) Ronald Fricke (State Museum of Natural History, Stuttgart, Germany) Antti Lappalainen (Finnish Game and Fisheries Research Institute), Atis Minde (Latvian Fish Resources Agency) Henn Ojaveer (Estonian Marine Institute) Wojciech Pelczarski (Sea Fisheries Institute, Poland) Rimantas Repečka (Institute of Ecology, Lithuania). Photographers: Visa Hietalahti p. cover, 7 top, 8 bottom Johnny Jensen p. 3 top, 3 bottom, 4 middle, 4 bottom, 5 top, 8 top, 9 top, 9 bottom Lauri Urho p. 4 top, 5 bottom Juhani Vaittinen p. 7 bottom Markku Varjo / LKA p. 10 top For bibliographic purposes this document should be cited as: HELCOM, 2006 Changing Communities of Baltic Coastal Fish Executive summary: Assessment of coastal fi sh in the Baltic Sea Balt. Sea Environ. Proc. No. 103 B Information included in this publication or extracts thereof is free for citing on the condition that the complete reference of the publication is given as stated above Copyright 2006 by the Baltic Marine Environment Protection Commission - Helsinki Commission - Design and layout: Bitdesign, Vantaa, Finland Printed by: Erweko Painotuote Oy, Finland ISSN 0357-2994 Coastal fi sh – a combination of freshwater and marine species Coastal fish communities are important components of Baltic Sea ecosystems. -

Stromatolites Below the Photic Zone in the Northern Arabian Sea Formed by Calcifying Chemotrophic Microbial Mats
Stromatolites below the photic zone in the northern Arabian Sea formed by calcifying chemotrophic microbial mats Tobias Himmler1*, Daniel Smrzka2, Jennifer Zwicker2, Sabine Kasten3,4, Russell S. Shapiro5, Gerhard Bohrmann1, and Jörn Peckmann2,6 1MARUM–Zentrum für Marine Umweltwissenschaften und Fachbereich Geowissenschaften, Universität Bremen, 28334 Bremen, Germany 2Department für Geodynamik und Sedimentologie, Erdwissenschaftliches Zentrum, Universität Wien, 1090 Wien, Austria 3Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung, 27570 Bremerhaven, Germany 4Fachbereich Geowissenschaften, Universität Bremen, 28359 Bremen, Germany 5Geological and Environmental Sciences Department, California State University–Chico, Chico, California 95929, USA 6Institut für Geologie, Centrum für Erdsystemforschung und Nachhaltigkeit, Universität Hamburg, 20146 Hamburg, Germany − − + 2− + ABSTRACT reduction: HS + NO3 + H + H2O → SO4 + NH4 (cf. Fossing et al., 1995; Chemosynthesis increases alkalinity and facilitates stromatolite Otte et al., 1999). The effect of this process, referred to as nitrate-driven growth at methane seeps in 731 m water depth within the oxygen sulfide oxidation (ND-SO), is amplified when it takes place near hotspots minimum zone (OMZ) in the northern Arabian Sea. Microbial fab- of SD-AOM (Siegert et al., 2013). Therefore, it has been hypothesized rics, including mineralized filament bundles resembling the sulfide- that fossilization of sulfide-oxidizing bacteria may occur during seep- oxidizing bacterium Thioploca, mineralized extracellular polymeric carbonate formation (Bailey et al., 2009). Yet, seep carbonates resulting substances, and fossilized rod-shaped and filamentous cells, all pre- from the putative interaction of sulfide-oxidizing bacteria with the SD- served in 13C-depleted authigenic carbonate, suggest that biofilm cal- AOM consortium have, to the best of our knowledge, only been recognized cification resulted from nitrate-driven sulfide oxidation (ND-SO) and in ancient seep deposits (Peckmann et al., 2004). -

Map of National and International River Basin Districts Version 29 October 2012
Map of National and International River Basin Districts Version 29 October 2012 -30° W -20° W -10° W 0° 10° E 20° E 30° E 40° E 50° E 60° E Azores (PT) k ar FI nm in Teno, F RU Atlantic Ocean NO NO Naatamojoki, s 0 100 m FI Paatsjoki ro km T T or T R ne o i r ve n Madeira (PT) r i Madeira (PT) FI o K e m i j o k i WHITE n j SEA o RU k FI d i n a l Atlantic Ocean 60° N d r Bothnian Bay NORWEGIAN o 0 100 N SE Oulujoki SEA km NO FI a Canaries (ES) i Canaries (ES) N g 1. La Palma n a l 2. El Hierro NO h 1 7 Moere NO e t 4 60° N d 3. La Gomera A o A K 5 n G and r o B u K V u o k s i 4. Tenerife e B c k 6 h l o e f y 2 3 Romsdal f o i m r Bothnian t p m o FI 5. Gran Canaria E h T o e f i n a j l F o 6. Fuertaventura Atlantic Sea i a e f a i k g n n i RU l n o j l - 7. Lanzarote 0 100 Ocean o a C Sogn u S S k n km e e i- d and G a a Glomma SE - O Fjordane Guadeloupe (FR) NO d Guadeloupe (FR) NO lan in f F Hordaland f o ul Caribbean age West G ass C E Sea e P Bay t Aland a up k North West s lo a t de a Islands ua r g Baltic Estonia EE E G I r e s t e t t o 0 100 g Agder a EE n km a i T K k Gauja a Rogaland ak r S d LV er n Martinique (FR) Scotland ag K LV D RU Martinique (FR) k a a South LV RU N S V tt e LV a e Baltic Neagh g n u a SE t Lielupe g t a a North Bann BALTIC LT v Caribbean A NORTH SEA LT BY a Western IE Jutland SEA LT Sea Solway and N LT L Swieza UK UK North- Funen e Tweet d Zealand m 0 10 North s umbria RU u Western IE e Bornholm Jarft a km IE IE Vidaa-Krusaa ly n W RU T n Eastern o a o g PL Eastern h DK Schlei/Trave e s 50° N n -

Historical Painting Techniques, Materials, and Studio Practice
Historical Painting Techniques, Materials, and Studio Practice PUBLICATIONS COORDINATION: Dinah Berland EDITING & PRODUCTION COORDINATION: Corinne Lightweaver EDITORIAL CONSULTATION: Jo Hill COVER DESIGN: Jackie Gallagher-Lange PRODUCTION & PRINTING: Allen Press, Inc., Lawrence, Kansas SYMPOSIUM ORGANIZERS: Erma Hermens, Art History Institute of the University of Leiden Marja Peek, Central Research Laboratory for Objects of Art and Science, Amsterdam © 1995 by The J. Paul Getty Trust All rights reserved Printed in the United States of America ISBN 0-89236-322-3 The Getty Conservation Institute is committed to the preservation of cultural heritage worldwide. The Institute seeks to advance scientiRc knowledge and professional practice and to raise public awareness of conservation. Through research, training, documentation, exchange of information, and ReId projects, the Institute addresses issues related to the conservation of museum objects and archival collections, archaeological monuments and sites, and historic bUildings and cities. The Institute is an operating program of the J. Paul Getty Trust. COVER ILLUSTRATION Gherardo Cibo, "Colchico," folio 17r of Herbarium, ca. 1570. Courtesy of the British Library. FRONTISPIECE Detail from Jan Baptiste Collaert, Color Olivi, 1566-1628. After Johannes Stradanus. Courtesy of the Rijksmuseum-Stichting, Amsterdam. Library of Congress Cataloguing-in-Publication Data Historical painting techniques, materials, and studio practice : preprints of a symposium [held at] University of Leiden, the Netherlands, 26-29 June 1995/ edited by Arie Wallert, Erma Hermens, and Marja Peek. p. cm. Includes bibliographical references. ISBN 0-89236-322-3 (pbk.) 1. Painting-Techniques-Congresses. 2. Artists' materials- -Congresses. 3. Polychromy-Congresses. I. Wallert, Arie, 1950- II. Hermens, Erma, 1958- . III. Peek, Marja, 1961- ND1500.H57 1995 751' .09-dc20 95-9805 CIP Second printing 1996 iv Contents vii Foreword viii Preface 1 Leslie A. -

Marine and Coastal Biodiversity: Draft Summary Report on the Description of Areas Meeting the Scientific Criteria for Ecological
CBD Distr. GENERAL CBD/SBSTTA/22/7/Add.1 3 April 2018 ORIGINAL: ENGLISH SUBSIDIARY BODY ON SCIENTIFIC, TECHNICAL AND TECHNOLOGICAL ADVICE Twenty-second meeting Montreal, Canada, 2-7 July 2018 Item 8 of the provisional agenda* MARINE AND COASTAL BIODIVERSITY DRAFT SUMMARY REPORT ON THE DESCRIPTION OF AREAS MEETING THE SCIENTIFIC CRITERIA FOR ECOLOGICALLY OR BIOLOGICALLY SIGNIFICANT MARINE AREAS Note by the Executive Secretary Addendum BACKGROUND 1. Pursuant to decision X/29, paragraph 36, decision XI/17, paragraph 12, decision XII/22, paragraph 6 and decision XIII/12, paragraph 8, the following two additional regional workshops were convened by the Executive Secretary of the Convention on Biological Diversity: (a) Black Sea and Caspian Sea (Baku, 24 to 29 April 2017);1 (b) Baltic Sea (Helsinki, 19 to 24 February 2018).2 2. The description of marine areas meeting the criteria for ecologically or biologically significant marine areas does not imply the expression of any opinion whatsoever concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Nor does it have economic or legal implications; it is strictly a scientific and technical exercise. 3. Pursuant to decision XI/17, paragraph 12, summaries of the results of these regional workshops are provided in tables 1 and 2 below, respectively, while full descriptions of how the areas meet the criteria for ecologically or biologically significant marine areas (EBSAs) are provided in the annexes to the respective reports of the workshops. * CBD/SBSTTA/22/1/Add.1. 1 Report contained in CBD/EBSA/WS/2017/1/3. -

International Council for the Exploration of the Sea FINLAND J. Lassig Institute of Marine Research C. H. 1982/L: L/Corr. Admini
\ International Council for C. H. 1982/L: l/Corr. the Exploration of the Sea Administrative Report Addendum 'BiologicalOceanography Committee FINLAND 6/0 J. Lassig Institute of Marine Research Phytoplankton, primary production, chlorophyll a and related parameters were studied every second week (twice during the ice period) at one station in the western part of the Gulf of Finland and at 15 stations in the entire Baltic Sea as stipula ted in theBaltic Monitoring Programme (Helsinki:Commission). Zooplankton was sampled (Hensen net) three times a month (once during the ice period) at two coastal stations in the Gulf of Finland, one station in the Archipelago Sea and one in the Bothnian Bay. Zooplankton was sampled (WP-2 net) at 26 stations in the entire Baltic Sea according to the Baltic Monitoring Programme·, I Benthic macrofauna communities were studied in the deep areas of the Baltic Sea. The stations of the Baltic Monitoring Pr~gramme were included in the survey. The produciton and decomposition of organie matter in the pela gial were studied in the Gulf of Finland in eooperation with Tvärminne Zoologieal Station of the University of Helsinki. Institute of Radiation Proteetion, Helsinki Benthos studies were carried out in the vieinity of two nuclear power plants, one in the Gulf of Finland and one in the Bothnian Bay. SampIes have been taken twiee at 9 stations at each plant. Phytoplankton, .ehlorophyll a and primary produetion studies were performed onee or twiee a month during the ice-free period around both plants. National Board of Waters, Water Research Office, Helsinki The influence of industrial pollution on the composition of th~ benthic macrofauna was studied in 4 areas in the Gulf of Finland, in 4 areas in~ the Bothnian Sea and in 3 areas in the Bothnian Bay. -

The in Vivo and in Vitro Assembly of Cell Walls in the Marine Coccolithophorid, Hymenomonas Carterae David Charles Flesch Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1977 The in vivo and in vitro assembly of cell walls in the marine coccolithophorid, Hymenomonas carterae David Charles Flesch Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Biology Commons Recommended Citation Flesch, David Charles, "The in vivo and in vitro assembly of cell walls in the marine coccolithophorid, Hymenomonas carterae " (1977). Retrospective Theses and Dissertations. 6115. https://lib.dr.iastate.edu/rtd/6115 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

Expression of Biomineralizationrelated Ion Transport Genes in Emiliania
Environmental Microbiology (2011) 13(12), 3250–3265 doi:10.1111/j.1462-2920.2011.02561.x Expression of biomineralization-related ion transport genes in Emiliania huxleyiemi_2561 3250..3265 Luke Mackinder,1,2* Glen Wheeler,1,3 Introduction Declan Schroeder,1 Peter von Dassow,4 Coccolithophores, unicellular calcifying marine algae, are Ulf Riebesell2 and Colin Brownlee1 a key component of today’s ocean playing an important 1Marine Biological Association of the UK, The role in nutrient and carbon cycling, as well as contribut- Laboratory, Citadel Hill, Plymouth PL1 2PB, UK. ing as much as half of current oceanic calcite production 2Leibniz Institute of Marine Science, IFM-GEOMAR, (Milliman, 1993). Emiliania huxleyi (division Haptophyta D-24105, Kiel, Germany. class Prymnesiophyceae) is the most abundant coccoli- 3Plymouth Marine Laboratory, Prospect Place, thophore species, producing extensive blooms at tem- Plymouth, UK. perate latitudes (Holligan et al., 1983; Fernandez et al., 4Departamento de Ecología, Facultad de Ciencias 1993; Holligan et al., 1993). Despite the obvious impor- Biológicas, Pontificia Universidad Católica de Chile, tance of coccolithophores, the function of coccoliths and Alameda #340, Santiago, Chile. the cellular processes underlying coccolith formation remain largely un-resolved. The intracellular production Summary of coccoliths of a size approaching the diameter of a single cell together with the rapid rate of coccolith pro- Biomineralization in the marine phytoplankton Emil- duction [~1 per hour (Paasche, 1962)] presents unique iania huxleyi is a stringently controlled intracellular questions for how Ca2+,H+ and inorganic carbon (C ) process. The molecular basis of coccolith production i balance is regulated in the cell. Mass balance equations is still relatively unknown although its importance in show that rapid rates of Ca2+ and C uptake must occur at global biogeochemical cycles and varying sensitivity i the cell plasma membrane (PM) and at the intracellular to increased pCO levels has been well documented.