Coccolithophores at Low Caco3 Saturation During Winter in the Bay of Biscay
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Overcalcified Forms of the Coccolithophore Emiliania Huxleyi in High CO2 Waters Are Not Pre-Adapted to Ocean Acidification
Biogeosciences Discuss., https://doi.org/10.5194/bg-2017-303 Manuscript under review for journal Biogeosciences Discussion started: 6 September 2017 c Author(s) 2017. CC BY 4.0 License. Overcalcified forms of the coccolithophore Emiliania huxleyi in high CO2 waters are not pre-adapted to ocean acidification. Peter von Dassow1,2,3*, Francisco Díaz-Rosas1,2, El Mahdi Bendif4, Juan-Diego Gaitán-Espitia5, Daniella Mella-Flores1, Sebastian Rokitta6, Uwe John6, and Rodrigo Torres7,8 5 1 Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile. 2 Instituto Milenio de Oceanografía de Chile. 3 UMI 3614, Evolutionary Biology and Ecology of Algae, CNRS-UPMC Sorbonne Universités, PUCCh, UACH. 4 Department of Plant Sciences, University of Oxford, OX1 3RB Oxford, UK. 5 CSIRO Oceans and Atmosphere, GPO Box 1538, Hobart 7001, TAS, Australia. 10 6 Marine Biogeosciences | PhytoChange Alfred Wegener Institute – Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany. 7 Centro de Investigación en Ecosistemas de la Patagonia (CIEP), Coyhaique, Chile. 8 Centro de Investigación: Dinámica de Ecosistemas marinos de Altas Latitudes (IDEAL), Punta Arenas, Chile. Correspondence to: Peter von Dassow ([email protected]) 15 Abstract. Marine multicellular organisms inhabiting waters with natural high fluctuations in pH appear more tolerant to acidification than conspecifics occurring in nearby stable waters, suggesting that environments of fluctuating pH hold genetic reservoirs for adaptation of key groups to ocean acidification (OA). The abundant and cosmopolitan calcifying phytoplankton Emiliania huxleyi exhibits a range of morphotypes with varying degrees of coccolith mineralization. We show that E. huxleyi populations in the naturally acidified upwelling waters of the Eastern South Pacific, where pH drops below 20 7.8 as is predicted for the global surface ocean by the year 2100, are dominated by exceptionally overcalcified morphotypes whose distal coccolith shield can be almost solid calcite. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -
Reduced Calcification of Marine Plankton in Response to Increased
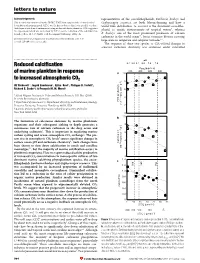
letters to nature Acknowledgements representatives of the coccolithophorids, Emiliania huxleyi and This research was sponsored by the EPSRC. T.W.F. ®rst suggested the electrochemical Gephyrocapsa oceanica, are both bloom-forming and have a deoxidation of titanium metal. G.Z.C. was the ®rst to observe that it was possible to reduce world-wide distribution. G. oceanica is the dominant coccolitho- thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested phorid in neritic environments of tropical waters9, whereas the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a. E. huxleyi, one of the most prominent producers of calcium carbonate in the world ocean10, forms extensive blooms covering Correspondence and requests for materials should be addressed to D. J. F. large areas in temperate and subpolar latitudes9,11. (e-mail: [email protected]). The response of these two species to CO2-related changes in seawater carbonate chemistry was examined under controlled ................................................................. pH Reduced calci®cation 8.4 8.2 8.1 8.0 7.9 7.8 PCO2 (p.p.m.v.) of marine plankton in response 200 400 600 800 a 10 to increased atmospheric CO2 ) 8 –1 Ulf Riebesell *, Ingrid Zondervan*, BjoÈrn Rost*, Philippe D. Tortell², d –1 Richard E. Zeebe*³ & FrancËois M. M. Morel² 6 * Alfred Wegener Institute for Polar and Marine Research, P.O. Box 120161, 4 D-27515 Bremerhaven, Germany mol C cell –13 ² Department of Geosciences & Department of Ecology and Evolutionary Biology, POC production Princeton University, Princeton, New Jersey 08544, USA (10 2 ³ Lamont-Doherty Earth Observatory, Columbia University, Palisades, New York 10964, USA 0 ............................................................................................................................................. -

Stromatolites Below the Photic Zone in the Northern Arabian Sea Formed by Calcifying Chemotrophic Microbial Mats
Stromatolites below the photic zone in the northern Arabian Sea formed by calcifying chemotrophic microbial mats Tobias Himmler1*, Daniel Smrzka2, Jennifer Zwicker2, Sabine Kasten3,4, Russell S. Shapiro5, Gerhard Bohrmann1, and Jörn Peckmann2,6 1MARUM–Zentrum für Marine Umweltwissenschaften und Fachbereich Geowissenschaften, Universität Bremen, 28334 Bremen, Germany 2Department für Geodynamik und Sedimentologie, Erdwissenschaftliches Zentrum, Universität Wien, 1090 Wien, Austria 3Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung, 27570 Bremerhaven, Germany 4Fachbereich Geowissenschaften, Universität Bremen, 28359 Bremen, Germany 5Geological and Environmental Sciences Department, California State University–Chico, Chico, California 95929, USA 6Institut für Geologie, Centrum für Erdsystemforschung und Nachhaltigkeit, Universität Hamburg, 20146 Hamburg, Germany − − + 2− + ABSTRACT reduction: HS + NO3 + H + H2O → SO4 + NH4 (cf. Fossing et al., 1995; Chemosynthesis increases alkalinity and facilitates stromatolite Otte et al., 1999). The effect of this process, referred to as nitrate-driven growth at methane seeps in 731 m water depth within the oxygen sulfide oxidation (ND-SO), is amplified when it takes place near hotspots minimum zone (OMZ) in the northern Arabian Sea. Microbial fab- of SD-AOM (Siegert et al., 2013). Therefore, it has been hypothesized rics, including mineralized filament bundles resembling the sulfide- that fossilization of sulfide-oxidizing bacteria may occur during seep- oxidizing bacterium Thioploca, mineralized extracellular polymeric carbonate formation (Bailey et al., 2009). Yet, seep carbonates resulting substances, and fossilized rod-shaped and filamentous cells, all pre- from the putative interaction of sulfide-oxidizing bacteria with the SD- served in 13C-depleted authigenic carbonate, suggest that biofilm cal- AOM consortium have, to the best of our knowledge, only been recognized cification resulted from nitrate-driven sulfide oxidation (ND-SO) and in ancient seep deposits (Peckmann et al., 2004). -

Historical Painting Techniques, Materials, and Studio Practice
Historical Painting Techniques, Materials, and Studio Practice PUBLICATIONS COORDINATION: Dinah Berland EDITING & PRODUCTION COORDINATION: Corinne Lightweaver EDITORIAL CONSULTATION: Jo Hill COVER DESIGN: Jackie Gallagher-Lange PRODUCTION & PRINTING: Allen Press, Inc., Lawrence, Kansas SYMPOSIUM ORGANIZERS: Erma Hermens, Art History Institute of the University of Leiden Marja Peek, Central Research Laboratory for Objects of Art and Science, Amsterdam © 1995 by The J. Paul Getty Trust All rights reserved Printed in the United States of America ISBN 0-89236-322-3 The Getty Conservation Institute is committed to the preservation of cultural heritage worldwide. The Institute seeks to advance scientiRc knowledge and professional practice and to raise public awareness of conservation. Through research, training, documentation, exchange of information, and ReId projects, the Institute addresses issues related to the conservation of museum objects and archival collections, archaeological monuments and sites, and historic bUildings and cities. The Institute is an operating program of the J. Paul Getty Trust. COVER ILLUSTRATION Gherardo Cibo, "Colchico," folio 17r of Herbarium, ca. 1570. Courtesy of the British Library. FRONTISPIECE Detail from Jan Baptiste Collaert, Color Olivi, 1566-1628. After Johannes Stradanus. Courtesy of the Rijksmuseum-Stichting, Amsterdam. Library of Congress Cataloguing-in-Publication Data Historical painting techniques, materials, and studio practice : preprints of a symposium [held at] University of Leiden, the Netherlands, 26-29 June 1995/ edited by Arie Wallert, Erma Hermens, and Marja Peek. p. cm. Includes bibliographical references. ISBN 0-89236-322-3 (pbk.) 1. Painting-Techniques-Congresses. 2. Artists' materials- -Congresses. 3. Polychromy-Congresses. I. Wallert, Arie, 1950- II. Hermens, Erma, 1958- . III. Peek, Marja, 1961- ND1500.H57 1995 751' .09-dc20 95-9805 CIP Second printing 1996 iv Contents vii Foreword viii Preface 1 Leslie A. -

The in Vivo and in Vitro Assembly of Cell Walls in the Marine Coccolithophorid, Hymenomonas Carterae David Charles Flesch Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1977 The in vivo and in vitro assembly of cell walls in the marine coccolithophorid, Hymenomonas carterae David Charles Flesch Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Biology Commons Recommended Citation Flesch, David Charles, "The in vivo and in vitro assembly of cell walls in the marine coccolithophorid, Hymenomonas carterae " (1977). Retrospective Theses and Dissertations. 6115. https://lib.dr.iastate.edu/rtd/6115 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

Expression of Biomineralizationrelated Ion Transport Genes in Emiliania
Environmental Microbiology (2011) 13(12), 3250–3265 doi:10.1111/j.1462-2920.2011.02561.x Expression of biomineralization-related ion transport genes in Emiliania huxleyiemi_2561 3250..3265 Luke Mackinder,1,2* Glen Wheeler,1,3 Introduction Declan Schroeder,1 Peter von Dassow,4 Coccolithophores, unicellular calcifying marine algae, are Ulf Riebesell2 and Colin Brownlee1 a key component of today’s ocean playing an important 1Marine Biological Association of the UK, The role in nutrient and carbon cycling, as well as contribut- Laboratory, Citadel Hill, Plymouth PL1 2PB, UK. ing as much as half of current oceanic calcite production 2Leibniz Institute of Marine Science, IFM-GEOMAR, (Milliman, 1993). Emiliania huxleyi (division Haptophyta D-24105, Kiel, Germany. class Prymnesiophyceae) is the most abundant coccoli- 3Plymouth Marine Laboratory, Prospect Place, thophore species, producing extensive blooms at tem- Plymouth, UK. perate latitudes (Holligan et al., 1983; Fernandez et al., 4Departamento de Ecología, Facultad de Ciencias 1993; Holligan et al., 1993). Despite the obvious impor- Biológicas, Pontificia Universidad Católica de Chile, tance of coccolithophores, the function of coccoliths and Alameda #340, Santiago, Chile. the cellular processes underlying coccolith formation remain largely un-resolved. The intracellular production Summary of coccoliths of a size approaching the diameter of a single cell together with the rapid rate of coccolith pro- Biomineralization in the marine phytoplankton Emil- duction [~1 per hour (Paasche, 1962)] presents unique iania huxleyi is a stringently controlled intracellular questions for how Ca2+,H+ and inorganic carbon (C ) process. The molecular basis of coccolith production i balance is regulated in the cell. Mass balance equations is still relatively unknown although its importance in show that rapid rates of Ca2+ and C uptake must occur at global biogeochemical cycles and varying sensitivity i the cell plasma membrane (PM) and at the intracellular to increased pCO levels has been well documented. -

University of Southampton Research Repository
University of Southampton Research Repository Copyright © and Moral Rights for this thesis and, where applicable, any accompanying data are retained by the author and/or other copyright owners. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis and the accompanying data cannot be reproduced or quoted extensively from without first obtaining permission in writing from the copyright holder/s. The content of the thesis and accompanying research data (where applicable) must not be changed in any way or sold commercially in any format or medium without the formal permission of the copyright holder/s. When referring to this thesis and any accompanying data, full bibliographic details must be given, e.g. Thesis: Author (Year of Submission) "Full thesis title", University of Southampton, name of the University Faculty or School or Department, PhD Thesis, pagination. Data: Author (Year) Title. URI [dataset] UNIVERSITY OF SOUTHAMPTON FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES School of Ocean and Earth Sciences Coccolithophores and Light: Photophysiology and Ecology amongst Different Species by Lucie Rebecca Daniels Submitted in partial fulfilment for the degree of Doctor of Philosophy August 2019 For Chris UNIVERSITY OF SOUTHAMPTON ABSTRACT FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES SCHOOL OF OCEAN AND EARTH SCIENCE Doctor of Philosophy Coccolithophores and Light: Photophysiology and Ecology amongst Different Species Lucie Rebecca Daniels The coccolithophores are a class of unicellular algae and are considered one of the key phytoplankton functional types. Regionally coccolithophores can contribute > 20 % of primary productivity and are major contributors to pelagic calcite production. Amongst the > 280 extant coccolithophore species there is remarkable diversity in morphology, and evidence that different species inhabit distinct ecological niches. -

Coccolith-Derived Turbidity and Hydrological Conditions in May In
Coccolith-derived turbidity and hydrological conditions in May in the Bay of Biscay Laurie Perrot, Francis Gohin, Diana Ruiz-Pino, Luis Lampert, Martin Huret, Aurélie Dessier, Pascale Malestroit, Christine Dupuy, Paul Bourriau To cite this version: Laurie Perrot, Francis Gohin, Diana Ruiz-Pino, Luis Lampert, Martin Huret, et al.. Coccolith-derived turbidity and hydrological conditions in May in the Bay of Biscay. Progress in Oceanography, Elsevier, 2018, 166, pp.42-53. 10.1016/j.pocean.2017.12.008. hal-01675026 HAL Id: hal-01675026 https://hal.archives-ouvertes.fr/hal-01675026 Submitted on 3 Jan 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Coccolith-derived turbidity and hydrological conditions in May in the Bay of Biscay 5 Laurie Perrot1, Francis Gohin1, Diana Ruiz-Pino2, Luis Lampert1, Martin Huret3, Aurélie Dessier4, Pascale Malestroit1, Christine Dupuy4, Paul Bourriau5 1 IFREMER, DYNECO-PELAGOS, Centre Ifremer de Brest CS 10070 , F-29280 Plouzane, France 2 Laboratoire LOCEAN-CNRS/IPSL, Université de Paris VI, Paris, F-75005, France 10 3 IFREMER, STH-LBH, Centre Ifremer de Brest CS 10070 , F-29280 Plouzane, France 4 Littoral Environnement et Sociétés (LIENSs), UMR 7266, CNRS-Université de La Rochelle, 2 rue Olympe de Gouges, F-17042 La Rochelle Cedex 01, France 5IFREMER, EMH, rue de l'Ile d'Yeu BP 21105, F-44311 Nantes Cedex 03, France 15 Corresponding author: Francis Gohin, IFREMER, DYNECO-PELAGOS, Centre Ifremer de Brest CS 10070 , F-29280 Plouzane, France . -

Coccolith Moulds in Sedimentary Organic Matter and Their Use in Palynofacies Analysis
J.micropalaeontol., 4 (2): 111-116, August 1985 Coccolith moulds in sedimentary organic matter and their use in palynofacies analysis D. J. BATTEN Department of Geology and Mineralogy, Marischal College, Aberdeen University, Aberdeen AB9 1AS, Scotland ABSTRACT - Imprints of coccoliths can be expected on palynomorphs and phytoclasts in Rhaetian and younger palynofacies. They resemble the hollows left by spheroidal pyrite but may commonly be distinguished on the basis of shape and by occasional marks which reflect the arrangement of the calcite laths of which they were composed. Monospecific blooms are suggested when they are abundant and their size and form is more or less constant. Those which are larger than average and bear arcuate grooves subparallel to their margins may indicate the former presence of coccospheres. The occurrence of coccolith moulds in pre-Quaternary palynofacies both indicates marine influence on the environment of deposition and provides evidence for the affinity of amorphous organic matter in problematical preparations which are swamped with this material and contain few palynomorphs. I NTRO DUCT I0N The identification of coccolith moulds in a palyno- Palynomorphs, cuticles and amorphous substances logical preparation can, therefore, provide a useful are among the various kinds of acid-resistant organic indication of marine influence through pre-Quaternary matter (kerogen) which frequently bear structures sequences that comprise sediments that were laid down indicating the former presence of minerals and other in variably saline to freshwater environments. They may microfossils in intimate association. Those left by pyrite also suggest a marine origin for amorphous matter of are the most abundant and widely recognised. -

Coccolithophores and Calcite Saturation State in the Baltic and Black Seas
Biogeosciences, 5, 485–494, 2008 www.biogeosciences.net/5/485/2008/ Biogeosciences © Author(s) 2008. This work is distributed under the Creative Commons Attribution 3.0 License. Coccolithophores and calcite saturation state in the Baltic and Black Seas T. Tyrrell1, B. Schneider2, A. Charalampopoulou1, and U. Riebesell3 1National Oceanography Centre, Southampton University, European Way, Southampton SO14 3ZH, UK 2Institut fur¨ Ostseeforschung Warnemunde,¨ Seestrasse 15, 18119 Rostock, Germany 3Leibniz-Institute of Marine Sciences, IFM-GEOMAR, Duesternbrooker Weg 20, 24105 Kiel, Germany Received: 25 September 2007 – Published in Biogeosciences Discuss.: 10 October 2007 Revised: 30 January 2008 – Accepted: 27 February 2008 – Published: 1 April 2008 Abstract. The Baltic and Black Seas are both brackish, that carbonate coccoliths (shells) (Merico et al., 2006). As far as is to say both have salinities intermediate between freshwa- inorganic precipitation and dissolution of calcium carbonate ter and seawater. The coccolithophore Emiliania huxleyi is are concerned, the tendency for these two processes to take abundant in one, the Black Sea, but absent from the other, place is governed by the saturation state: the Baltic Sea. Here we present summertime coccolithophore = [ 2−] [ 2+] measurements confirming this difference, as well as data on CO3 . Ca /Ksp (1) the calcium carbonate saturation state of the Baltic Sea. We find that the Baltic Sea becomes undersaturated (or nearly with inorganic precipitation increasingly likely and/or rapid so) in winter, with respect to both the aragonite and calcite as increases further above 1.0, and dissolution increasingly likely and/or rapid as decreases further below 1.0. mineral forms of CaCO3. Data for the Black Sea are more limited, but it appears to remain strongly supersaturated year- If biogenic calcification were to be controlled in the same round. -

Modeling Coccolithophores in the Global Oceans
Modeling Coccolithophores in the Global Oceans Watson W. Gregg Global Modeling and Assimilation Office NASA/Goddard Space Flight Center Greenbelt, MD 20771 [email protected] Nancy W. Casey Science Systems and Applications, Inc. Lanham, MD 20706 [email protected] Deep-Sea Research II Special Issue Submitted March, 2006 Revised May, 2006 Abstract Coccolithophores are important ecological and geochemical components of the global oceans. A global three-dimensional model was used to simulate their distributions in a multi- phytoplankton context. The realism of the simulation was supported by comparisons of model surface nutrients and total chlorophyll with in situ and satellite observations. Nitrate, silica, and dissolved iron surface distributions were positively correlated with in situ data across major oceanographic basins. Global annual departures were +18.9% for nitrate (model high), +5.4% for silica, and +45.0% for iron. Total surface chlorophyll was also positively correlated with satellite and in situ data sets across major basins. Global annual departures were -8.0% with SeaWiFS (model low), +1.1% with Aqua, and -17.1% with in situ data. Global annual primary production estimates were within 1% and 9% of estimates derived from SeaWiFS and Aqua, respectively, using a common primary production algorithm. Coccolithophore annual mean relative abundances were 2.6% lower than observations, but were positively correlated across basins. Two of the other three phytoplankton groups, diatoms and cyanobacteria, were also positively correlated with observations. Distributions of coccolithophores were dependent upon interactions and competition with the other phytoplankton groups. In this model coccolithophores had a competitive advantage over diatoms and chlorophytes by virtue of a greater ability to utilize nutrients and light at low values.