In Marinobacter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Overcalcified Forms of the Coccolithophore Emiliania Huxleyi in High CO2 Waters Are Not Pre-Adapted to Ocean Acidification
Biogeosciences Discuss., https://doi.org/10.5194/bg-2017-303 Manuscript under review for journal Biogeosciences Discussion started: 6 September 2017 c Author(s) 2017. CC BY 4.0 License. Overcalcified forms of the coccolithophore Emiliania huxleyi in high CO2 waters are not pre-adapted to ocean acidification. Peter von Dassow1,2,3*, Francisco Díaz-Rosas1,2, El Mahdi Bendif4, Juan-Diego Gaitán-Espitia5, Daniella Mella-Flores1, Sebastian Rokitta6, Uwe John6, and Rodrigo Torres7,8 5 1 Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile. 2 Instituto Milenio de Oceanografía de Chile. 3 UMI 3614, Evolutionary Biology and Ecology of Algae, CNRS-UPMC Sorbonne Universités, PUCCh, UACH. 4 Department of Plant Sciences, University of Oxford, OX1 3RB Oxford, UK. 5 CSIRO Oceans and Atmosphere, GPO Box 1538, Hobart 7001, TAS, Australia. 10 6 Marine Biogeosciences | PhytoChange Alfred Wegener Institute – Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany. 7 Centro de Investigación en Ecosistemas de la Patagonia (CIEP), Coyhaique, Chile. 8 Centro de Investigación: Dinámica de Ecosistemas marinos de Altas Latitudes (IDEAL), Punta Arenas, Chile. Correspondence to: Peter von Dassow ([email protected]) 15 Abstract. Marine multicellular organisms inhabiting waters with natural high fluctuations in pH appear more tolerant to acidification than conspecifics occurring in nearby stable waters, suggesting that environments of fluctuating pH hold genetic reservoirs for adaptation of key groups to ocean acidification (OA). The abundant and cosmopolitan calcifying phytoplankton Emiliania huxleyi exhibits a range of morphotypes with varying degrees of coccolith mineralization. We show that E. huxleyi populations in the naturally acidified upwelling waters of the Eastern South Pacific, where pH drops below 20 7.8 as is predicted for the global surface ocean by the year 2100, are dominated by exceptionally overcalcified morphotypes whose distal coccolith shield can be almost solid calcite. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -
Reduced Calcification of Marine Plankton in Response to Increased
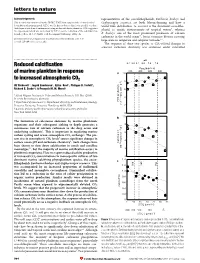
letters to nature Acknowledgements representatives of the coccolithophorids, Emiliania huxleyi and This research was sponsored by the EPSRC. T.W.F. ®rst suggested the electrochemical Gephyrocapsa oceanica, are both bloom-forming and have a deoxidation of titanium metal. G.Z.C. was the ®rst to observe that it was possible to reduce world-wide distribution. G. oceanica is the dominant coccolitho- thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested phorid in neritic environments of tropical waters9, whereas the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a. E. huxleyi, one of the most prominent producers of calcium carbonate in the world ocean10, forms extensive blooms covering Correspondence and requests for materials should be addressed to D. J. F. large areas in temperate and subpolar latitudes9,11. (e-mail: [email protected]). The response of these two species to CO2-related changes in seawater carbonate chemistry was examined under controlled ................................................................. pH Reduced calci®cation 8.4 8.2 8.1 8.0 7.9 7.8 PCO2 (p.p.m.v.) of marine plankton in response 200 400 600 800 a 10 to increased atmospheric CO2 ) 8 –1 Ulf Riebesell *, Ingrid Zondervan*, BjoÈrn Rost*, Philippe D. Tortell², d –1 Richard E. Zeebe*³ & FrancËois M. M. Morel² 6 * Alfred Wegener Institute for Polar and Marine Research, P.O. Box 120161, 4 D-27515 Bremerhaven, Germany mol C cell –13 ² Department of Geosciences & Department of Ecology and Evolutionary Biology, POC production Princeton University, Princeton, New Jersey 08544, USA (10 2 ³ Lamont-Doherty Earth Observatory, Columbia University, Palisades, New York 10964, USA 0 ............................................................................................................................................. -

1 Marinobacter Hydrocarbonoclasticus
International Journal of Systematic Bacteriology (1998), 48, 1445-1 448 Printed in Great Britain ~ -~~~~~~ 1- Transfer of Pseudomonas nautica to L -~ 1 Marinobacter hydrocarbonoclasticus Cathrin Sproer, Elke Lang, Petra Hobeck, Jutta Burghardt, Erko Stackebrandt and B. J. Tindall Author for correspondence: B. J. Tindall. Tel: +49 531 2616 224. Fax: +49 531 2616 418. e-mail: bti@ gbf.de ~ DSMZ-Deutsche Sammlung A combination of genotypic and phenotypic properties (a polyphasic "On Mikroorganismen und taxonomic approach) was used to determine the relatedness between the type Zellkulturen GmbH, Mascheroder Weg 1b, strains of Pseudomonas nautica Bauman et a/. 1982 and Marinobacter D-38124 Braunschweig, hydrocarbonoc/asticusGauthier et a/. 1992, which were originally found to be Germany highly related by partial 16s rDNA sequence analysis. Analysis of genotypic properties, such as comparison of the almost complete 165 rDNA sequences, base composition of the total genomic DNA and DNA-DNA hybridization revealed that the two strains were highly similar and should be considered members of the same species. The phenotypic properties, such as the physiology and chemotaxonomic data (i.e. fatty acid composition, polar lipid patterns and respiratory lipoquinone content), confirmed the genotypic evaluation, and has lead to the proposal for a unification of the two species, Pseudomonas nautica (DSM 50418') and Marinobacter hydrocarbonodasticus (DSM 8798T)as Marinobacter hydrocarbonoclasticus. Keywords: Marinobacter hil~drocarbonoclasticus, Pseudomonas nautica, 16s rRN A sequence, chemotaxonomy, taxonomy Baumann et a/. (1972) described aerobic, oxidase- to members of the Pseudomonas aeruginosa rRNA positive. Gram-negative and motile strains which branch of the rRNA superfamily I (De Ley, 1978), showed a high degree of physiological similarity, but with the remaining species being transferred to other were different from other members of the genus genera, e .g . -

1 Microbial Transformations of Organic Chemicals in Produced Fluid From
Microbial transformations of organic chemicals in produced fluid from hydraulically fractured natural-gas wells Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Morgan V. Evans Graduate Program in Environmental Science The Ohio State University 2019 Dissertation Committee Professor Paula Mouser, Advisor Professor Gil Bohrer, Co-Advisor Professor Matthew Sullivan, Member Professor Ilham El-Monier, Member Professor Natalie Hull, Member 1 Copyrighted by Morgan Volker Evans 2019 2 Abstract Hydraulic fracturing and horizontal drilling technologies have greatly improved the production of oil and natural-gas from previously inaccessible non-permeable rock formations. Fluids comprised of water, chemicals, and proppant (e.g., sand) are injected at high pressures during hydraulic fracturing, and these fluids mix with formation porewaters and return to the surface with the hydrocarbon resource. Despite the addition of biocides during operations and the brine-level salinities of the formation porewaters, microorganisms have been identified in input, flowback (days to weeks after hydraulic fracturing occurs), and produced fluids (months to years after hydraulic fracturing occurs). Microorganisms in the hydraulically fractured system may have deleterious effects on well infrastructure and hydrocarbon recovery efficiency. The reduction of oxidized sulfur compounds (e.g., sulfate, thiosulfate) to sulfide has been associated with both well corrosion and souring of natural-gas, and proliferation of microorganisms during operations may lead to biomass clogging of the newly created fractures in the shale formation culminating in reduced hydrocarbon recovery. Consequently, it is important to elucidate microbial metabolisms in the hydraulically fractured ecosystem. -

Stromatolites Below the Photic Zone in the Northern Arabian Sea Formed by Calcifying Chemotrophic Microbial Mats
Stromatolites below the photic zone in the northern Arabian Sea formed by calcifying chemotrophic microbial mats Tobias Himmler1*, Daniel Smrzka2, Jennifer Zwicker2, Sabine Kasten3,4, Russell S. Shapiro5, Gerhard Bohrmann1, and Jörn Peckmann2,6 1MARUM–Zentrum für Marine Umweltwissenschaften und Fachbereich Geowissenschaften, Universität Bremen, 28334 Bremen, Germany 2Department für Geodynamik und Sedimentologie, Erdwissenschaftliches Zentrum, Universität Wien, 1090 Wien, Austria 3Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung, 27570 Bremerhaven, Germany 4Fachbereich Geowissenschaften, Universität Bremen, 28359 Bremen, Germany 5Geological and Environmental Sciences Department, California State University–Chico, Chico, California 95929, USA 6Institut für Geologie, Centrum für Erdsystemforschung und Nachhaltigkeit, Universität Hamburg, 20146 Hamburg, Germany − − + 2− + ABSTRACT reduction: HS + NO3 + H + H2O → SO4 + NH4 (cf. Fossing et al., 1995; Chemosynthesis increases alkalinity and facilitates stromatolite Otte et al., 1999). The effect of this process, referred to as nitrate-driven growth at methane seeps in 731 m water depth within the oxygen sulfide oxidation (ND-SO), is amplified when it takes place near hotspots minimum zone (OMZ) in the northern Arabian Sea. Microbial fab- of SD-AOM (Siegert et al., 2013). Therefore, it has been hypothesized rics, including mineralized filament bundles resembling the sulfide- that fossilization of sulfide-oxidizing bacteria may occur during seep- oxidizing bacterium Thioploca, mineralized extracellular polymeric carbonate formation (Bailey et al., 2009). Yet, seep carbonates resulting substances, and fossilized rod-shaped and filamentous cells, all pre- from the putative interaction of sulfide-oxidizing bacteria with the SD- served in 13C-depleted authigenic carbonate, suggest that biofilm cal- AOM consortium have, to the best of our knowledge, only been recognized cification resulted from nitrate-driven sulfide oxidation (ND-SO) and in ancient seep deposits (Peckmann et al., 2004). -

Historical Painting Techniques, Materials, and Studio Practice
Historical Painting Techniques, Materials, and Studio Practice PUBLICATIONS COORDINATION: Dinah Berland EDITING & PRODUCTION COORDINATION: Corinne Lightweaver EDITORIAL CONSULTATION: Jo Hill COVER DESIGN: Jackie Gallagher-Lange PRODUCTION & PRINTING: Allen Press, Inc., Lawrence, Kansas SYMPOSIUM ORGANIZERS: Erma Hermens, Art History Institute of the University of Leiden Marja Peek, Central Research Laboratory for Objects of Art and Science, Amsterdam © 1995 by The J. Paul Getty Trust All rights reserved Printed in the United States of America ISBN 0-89236-322-3 The Getty Conservation Institute is committed to the preservation of cultural heritage worldwide. The Institute seeks to advance scientiRc knowledge and professional practice and to raise public awareness of conservation. Through research, training, documentation, exchange of information, and ReId projects, the Institute addresses issues related to the conservation of museum objects and archival collections, archaeological monuments and sites, and historic bUildings and cities. The Institute is an operating program of the J. Paul Getty Trust. COVER ILLUSTRATION Gherardo Cibo, "Colchico," folio 17r of Herbarium, ca. 1570. Courtesy of the British Library. FRONTISPIECE Detail from Jan Baptiste Collaert, Color Olivi, 1566-1628. After Johannes Stradanus. Courtesy of the Rijksmuseum-Stichting, Amsterdam. Library of Congress Cataloguing-in-Publication Data Historical painting techniques, materials, and studio practice : preprints of a symposium [held at] University of Leiden, the Netherlands, 26-29 June 1995/ edited by Arie Wallert, Erma Hermens, and Marja Peek. p. cm. Includes bibliographical references. ISBN 0-89236-322-3 (pbk.) 1. Painting-Techniques-Congresses. 2. Artists' materials- -Congresses. 3. Polychromy-Congresses. I. Wallert, Arie, 1950- II. Hermens, Erma, 1958- . III. Peek, Marja, 1961- ND1500.H57 1995 751' .09-dc20 95-9805 CIP Second printing 1996 iv Contents vii Foreword viii Preface 1 Leslie A. -

Marinobacter Aquaeolei Sp. Nov., a Halophilic Bacterium Isolated from a Vietnamese Oil- Producing Well
lnternational Journal of Systematic Bacteriology (1999), 49, 367-375 Printed in Great Britain Marinobacter aquaeolei sp. nov., a halophilic bacterium isolated from a Vietnamese oil- producing well Nguyen 6. Huu,' Ewald B. M. Denner,' Dang T. C. Ha,' Gerhard Wanner3 and Helga Stan-Lotter4 Author for correspondence: Helga Stan-Lotter. Tel: +43 662 8044 5756. Fax: +43 662 8044 144. e-mail : helgastan-lo tter @ sbg.ac.at 1 Institute of Biotechnology, Several strains of moderately halophilic and mesophilic bacteria were isolated National Center for at the head of an oil-producing well on an offshore platform in southern Natural Science and Technology, Nghia do, Tu Vietnam. Cells were Gram-negative, non-spore-forming, rod-shaped and motile liem, Hanoi, Vietnam by means of a polar f lagellum. Growth occurred at NaCl concentrations 2 lnstitut fur Mikrobiologie between 0 and 20%; the optimum was 5% NaCl. One strain, which was und Genetik, Universitdt designated VT8l, could degrade n-hexadecane, pristane and some crude oil Wien, Dr Bohrgasse 9, components. It grew anaerobically in the presence of nitrate on succinate, A-1030 Wien, Austria citrate or acetate, but not on glucose. Several organic acids and amino acids 3 Botanisches lnstitut der were utilized as sole carbon and energy sources. The major components of its Universitdt Munchen, Menzinger Str. 67, D-80638 cellular fatty acids were Clzr0 3-OH, c16:1 09c, c16:o and C18:1 w9c. The DNA G+C Munchen, Germany content was 557 mol0/o. 165 rDNA sequence analysis indicated that strain VT8T 4 lnstitut fur Genetik und was closely related to Marinobacter sp. -

Marinobacter Maroccanus Sp. Nov., a Moderately Halophilic Bacterium Isolated from a Saline Soil
Full PDF (including article, references, figures, tables) Click here to download Full PDF (including article, references, figures, tables) Marinobacter maroccanus.pdf 1 Marinobacter maroccanus sp. nov., a moderately halophilic bacterium 2 isolated from a saline soil 3 4 Nadia Boujida,1† Montserrat Palau,2† Saoulajan Charfi,1 Àngels Manresa,2 Nadia Skali 5 Senhaji,1 Jamal Abrini,1 David Miñana-Galbis2* 6 7 Author affiliations: 1Biotechnology and Applied Microbiology Research Group, 8 Department of Biology, Faculty of Sciences, University Abdelmalek Essaâdi, BP2121, 9 93002 Tetouan, Morocco; 2Secció de Microbiologia, Dept. Biologia, Sanitat i Medi 10 Ambient, Facultat de Farmàcia i Ciències de l'Alimentació, Universitat de Barcelona, Av. 11 Joan XXIII, 27-31, 08028 Barcelona, Catalonia, Spain. 12 13 †These authors contributed equally to this work. 14 15 *Correspondence: David Miñana-Galbis, [email protected] 16 17 Keywords: Marinobacter maroccanus sp. nov.; halophilic bacterium. 18 19 The GenBank/EMBL/DDBJ accession numbers for the 16S rRNA and rpoD gene 20 sequences and the whole genome shotgun project of strain N4T are MG563241, 21 MG551593, and PSSX01000000, respectively. 22 1 23 Abstract 24 25 During the taxonomic investigation of exopolymer producing halophilic bacteria, a rod- 26 shaped, motile, Gram-stain-negative, aerobic, halophilic bacterium, designated strain 27 N4T, was isolated from a natural saline soil located in the northern Morocco. The optimal 28 growth of the isolate was at 30–37 ºC and at pH 6.0–9.0, in the presence of 5–7% (w/v) 29 NaCl. Useful tests for the phenotypic differentiation of strain N4T from other Marinobacter 30 species included α-chymotrypsin and α-glucosidase activities and the carbohydrate T 31 assimilation profile. -

The in Vivo and in Vitro Assembly of Cell Walls in the Marine Coccolithophorid, Hymenomonas Carterae David Charles Flesch Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1977 The in vivo and in vitro assembly of cell walls in the marine coccolithophorid, Hymenomonas carterae David Charles Flesch Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Biology Commons Recommended Citation Flesch, David Charles, "The in vivo and in vitro assembly of cell walls in the marine coccolithophorid, Hymenomonas carterae " (1977). Retrospective Theses and Dissertations. 6115. https://lib.dr.iastate.edu/rtd/6115 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

Expression of Biomineralizationrelated Ion Transport Genes in Emiliania
Environmental Microbiology (2011) 13(12), 3250–3265 doi:10.1111/j.1462-2920.2011.02561.x Expression of biomineralization-related ion transport genes in Emiliania huxleyiemi_2561 3250..3265 Luke Mackinder,1,2* Glen Wheeler,1,3 Introduction Declan Schroeder,1 Peter von Dassow,4 Coccolithophores, unicellular calcifying marine algae, are Ulf Riebesell2 and Colin Brownlee1 a key component of today’s ocean playing an important 1Marine Biological Association of the UK, The role in nutrient and carbon cycling, as well as contribut- Laboratory, Citadel Hill, Plymouth PL1 2PB, UK. ing as much as half of current oceanic calcite production 2Leibniz Institute of Marine Science, IFM-GEOMAR, (Milliman, 1993). Emiliania huxleyi (division Haptophyta D-24105, Kiel, Germany. class Prymnesiophyceae) is the most abundant coccoli- 3Plymouth Marine Laboratory, Prospect Place, thophore species, producing extensive blooms at tem- Plymouth, UK. perate latitudes (Holligan et al., 1983; Fernandez et al., 4Departamento de Ecología, Facultad de Ciencias 1993; Holligan et al., 1993). Despite the obvious impor- Biológicas, Pontificia Universidad Católica de Chile, tance of coccolithophores, the function of coccoliths and Alameda #340, Santiago, Chile. the cellular processes underlying coccolith formation remain largely un-resolved. The intracellular production Summary of coccoliths of a size approaching the diameter of a single cell together with the rapid rate of coccolith pro- Biomineralization in the marine phytoplankton Emil- duction [~1 per hour (Paasche, 1962)] presents unique iania huxleyi is a stringently controlled intracellular questions for how Ca2+,H+ and inorganic carbon (C ) process. The molecular basis of coccolith production i balance is regulated in the cell. Mass balance equations is still relatively unknown although its importance in show that rapid rates of Ca2+ and C uptake must occur at global biogeochemical cycles and varying sensitivity i the cell plasma membrane (PM) and at the intracellular to increased pCO levels has been well documented. -

University of Southampton Research Repository
University of Southampton Research Repository Copyright © and Moral Rights for this thesis and, where applicable, any accompanying data are retained by the author and/or other copyright owners. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis and the accompanying data cannot be reproduced or quoted extensively from without first obtaining permission in writing from the copyright holder/s. The content of the thesis and accompanying research data (where applicable) must not be changed in any way or sold commercially in any format or medium without the formal permission of the copyright holder/s. When referring to this thesis and any accompanying data, full bibliographic details must be given, e.g. Thesis: Author (Year of Submission) "Full thesis title", University of Southampton, name of the University Faculty or School or Department, PhD Thesis, pagination. Data: Author (Year) Title. URI [dataset] UNIVERSITY OF SOUTHAMPTON FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES School of Ocean and Earth Sciences Coccolithophores and Light: Photophysiology and Ecology amongst Different Species by Lucie Rebecca Daniels Submitted in partial fulfilment for the degree of Doctor of Philosophy August 2019 For Chris UNIVERSITY OF SOUTHAMPTON ABSTRACT FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES SCHOOL OF OCEAN AND EARTH SCIENCE Doctor of Philosophy Coccolithophores and Light: Photophysiology and Ecology amongst Different Species Lucie Rebecca Daniels The coccolithophores are a class of unicellular algae and are considered one of the key phytoplankton functional types. Regionally coccolithophores can contribute > 20 % of primary productivity and are major contributors to pelagic calcite production. Amongst the > 280 extant coccolithophore species there is remarkable diversity in morphology, and evidence that different species inhabit distinct ecological niches.