Enter Your Title Here in All Capital Letters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Food and Drink War and Peace
NATIONAL MUSEUM Of ThE AMERICAN INDIANSUMMER 2018 FooD AND DrINk Healthy Eating ANd SOvEREIgNTy ThE Persistence Of ChIChA + WAr AND PeAce hUMbLE Hero Of d-Day NAvAjO Treaty Of 1868 JOIN TODAY FOR ONLY $25 – DON’T MISS ANOTHER ISSUE! NATIONAL MUSEUM of the AMERICAN INDIANFALL 2010 DARK WATERS THE FORMIDABLE ART OF MICHAEL BELMORE EXPLAINING ANDEAN DESIGN THE REMARKABLE LARANCE SPECIAL ISSUE ............................... FAMILY DECEMBER INDIANS ON THE POST ART OFFICE MARKETS WALLS + A NEW VANTAGE POINT ON CONTEMPORARY ARTISTS JOIN TODAY AND LET THE MUSEUM COME TO YOU! BECOME A MEMBER OF THE NATIONAL • 20% discount on all books purchased MUSEUM OF THE AMERICAN INDIAN from the NMAI web site FOR JUST $25 AND YOU’LL RECEIVE: • 10% discount on all purchases from • FREE 1 year subscription to our exclusive, the Mitsitam Café and all NMAI and full-color quarterly publication, American Smithsonian Museum Stores Indian magazine • Permanent Listing on NMAI’s electronic • Preferred Entry to the NMAI Mall Member and Donor Scroll Museum at peak visitor times Join online at www.AmericanIndian.si.edu or call toll free at 800-242-NMAI (6624) or simply mail your check for $25 to NMAI, Member Services PO Box 23473, Washington DC 20026-3473 SMITHSONIAN INSTITUTION 17 NMAI_FALL15.indd 16 2015-07-17 1:00 PM Contents SUMMER 2018 VOL. 19 NO. 2 18 10 ON THE COVER NATIONAL MUSEUM O F THE AMERICAN Traditional food and drink continue to sustain Indigenous identity and cultural (and political) survival. This richly carved Inka qero (wooden drinking cup) shows a mule team hauling house SUMMER 2018 beams to the highlands as a Native woman offers a INDIAN drink of chicha to the mule drivers. -

Prezentacja Programu Powerpoint
Discover new opportunities „Grana” Sp. z o.o. company and product offer presentation Company | About Grana company • One of the world’s largest producers of instant beverages made from cereals and chicory • We have been making cereal and chicory-based beverages for 100 years • We offer primarily top quality products developed thanks to our wealth of experience and state-of-the-art technologies. • We are a unit of the German Group Cafea - it is Grana in numbers one of the biggest companies in the world specialized in the production of instant coffees for the private label market. - 26 000 sq. m plot area - 25 000 sq. m production area • We cooperate with clients from all over the - 6 100 sq. m. warehouse area world and our products are available in stores across Europe as well as the US, Canada, Japan - 290 employees and Malaysia. Production process | Production process Producing an instant beverage is a technologically demanding task but it can be simply described as follows. The best quality ingredients undergo the following processes: roasting and water extraction (which enables obtaining the liquid essence of the ingredients). Next, the extract is dried. After drying we obtain a quick and easy-to-prepare powder/granules with a delicate taste and aroma and amber-coffee colour. Selection & delivery Roasting Extraction Drying Packaging Raw materials, mainly One of the most Roasted semi- Liquid essence is Finished products cereals and chicory – important production products undergo dried by hot air in can be packed in used for production processes during water extraction one of the two jars, cans, bags, come from selected which raw materials process during which drying towers. -

Great Food, Great Stories from Korea
GREAT FOOD, GREAT STORIE FOOD, GREAT GREAT A Tableau of a Diamond Wedding Anniversary GOVERNMENT PUBLICATIONS This is a picture of an older couple from the 18th century repeating their wedding ceremony in celebration of their 60th anniversary. REGISTRATION NUMBER This painting vividly depicts a tableau in which their children offer up 11-1541000-001295-01 a cup of drink, wishing them health and longevity. The authorship of the painting is unknown, and the painting is currently housed in the National Museum of Korea. Designed to help foreigners understand Korean cuisine more easily and with greater accuracy, our <Korean Menu Guide> contains information on 154 Korean dishes in 10 languages. S <Korean Restaurant Guide 2011-Tokyo> introduces 34 excellent F Korean restaurants in the Greater Tokyo Area. ROM KOREA GREAT FOOD, GREAT STORIES FROM KOREA The Korean Food Foundation is a specialized GREAT FOOD, GREAT STORIES private organization that searches for new This book tells the many stories of Korean food, the rich flavors that have evolved generation dishes and conducts research on Korean cuisine after generation, meal after meal, for over several millennia on the Korean peninsula. in order to introduce Korean food and culinary A single dish usually leads to the creation of another through the expansion of time and space, FROM KOREA culture to the world, and support related making it impossible to count the exact number of dishes in the Korean cuisine. So, for this content development and marketing. <Korean Restaurant Guide 2011-Western Europe> (5 volumes in total) book, we have only included a selection of a hundred or so of the most representative. -

Final Report Specialty Coffee and Cocoa
Value Chain Analysis CBI Integrated Country Programme Final Report Specialty coffee and cocoa Contact: Udo Censkowsky +49-89-82075902 [email protected] www.organic-services.com 1 Organic Services - committed to creating value www.organic-services.com Content 1. Introduction ....................................................................................................... 3 2. Market demand in the European Union ............................................................ 4 2.1. Global situation and European imports ....................................................... 4 2.2. Domestic market trends in Peru ................................................................ 10 2.3. EU import requirements ............................................................................ 11 3. Coffee & cacao value chain analysis .............................................................. 14 3.1. Governance of the coffee and cocoa sector ............................................. 14 3.2. Status-Quo production and trends ............................................................ 15 3.3. Value Chain Analysis ................................................................................ 19 4. Number of Peruvian companies ...................................................................... 25 5. Risk assessment and opportunities ................................................................ 26 6. Role of stakeholders in a CBI country programme.......................................... 33 7. Corporate Social Responsibility -

Greener Products; Earth Week Information
A PUBLICATION OF WILLY STREET CO-OP, MADISON, WI VOLUME 45 • ISSUE 4 •APRIL 2018 IN THIS ISSUE: Run for the Board; Greener Products; Earth Week Information; West Expansion Update; and More! PAID PRSRT STD PRSRT U.S. POSTAGE POSTAGE U.S. MADISON, WI MADISON, PERMIT NO. 1723 NO. PERMIT 1457 E. Washington Ave • Madison, WI 53703 Ave 1457 E. Washington POSTMASTER: DATED MATERIAL POSTMASTER: DATED CHANGE SERVICE REQUESTED CHANGE SERVICE WILLY STREET CO-OP MISSION STATEMENT The Williamson Street Grocery Co-op is an economically and environmentally sustainable, coop- READER eratively owned grocery business that serves the needs of its Owners Published monthly by Willy Street Co-op and employees. We are a cor- East: 1221 Williamson Street, Madison, WI 53703, 608-251-6776 nerstone of a vibrant community West: 6825 University Ave, Middleton, WI 53562, 608-284-7800 in south-central Wisconsin that North: 2817 N. Sherman Ave, Madison, WI 53704, 608-471-4422 provides fairly priced goods and Central Office: 1457 E. Washington Ave, Madison, WI 53703, 608-251-0884 services while supporting local EDITOR & LAYOUT: Liz Wermcrantz and organic suppliers. ADVERTISING: Liz Wermcrantz COVER DESIGN: Hallie Zillman-Bouche SALE FLYER DESIGN: Hallie Zillman-Bouche GRAPHICS: Hallie Zillman-Bouche SALE FLYER LAYOUT: Liz Wermcrantz WILLY STREET CO-OP PRINTING: Wingra Printing Group BOARD OF DIRECTORS The Willy Street Co-op Reader is the monthly communications link among the Co-op Board, staff and Owners. It provides information about the Co-op’s services Holly Fearing, President and business as well as about cooking, nutrition, health, sustainable agriculture and Patricia Butler more. -

FIC-Prop-65-Notice-Reporter.Pdf
FIC Proposition 65 Food Notice Reporter (Current as of 9/25/2021) A B C D E F G H Date Attorney Alleged Notice General Manufacturer Product of Amended/ Additional Chemical(s) 60 day Notice Link was Case /Company Concern Withdrawn Notice Detected 1 Filed Number Sprouts VeggIe RotInI; Sprouts FruIt & GraIn https://oag.ca.gov/system/fIl Sprouts Farmers Cereal Bars; Sprouts 9/24/21 2021-02369 Lead es/prop65/notIces/2021- Market, Inc. SpInach FettucIne; 02369.pdf Sprouts StraIght Cut 2 Sweet Potato FrIes Sprouts Pasta & VeggIe https://oag.ca.gov/system/fIl Sprouts Farmers 9/24/21 2021-02370 Sauce; Sprouts VeggIe Lead es/prop65/notIces/2021- Market, Inc. 3 Power Bowl 02370.pdf Dawn Anderson, LLC; https://oag.ca.gov/system/fIl 9/24/21 2021-02371 Sprouts Farmers OhI Wholesome Bars Lead es/prop65/notIces/2021- 4 Market, Inc. 02371.pdf Brad's Raw ChIps, LLC; https://oag.ca.gov/system/fIl 9/24/21 2021-02372 Sprouts Farmers Brad's Raw ChIps Lead es/prop65/notIces/2021- 5 Market, Inc. 02372.pdf Plant Snacks, LLC; Plant Snacks Vegan https://oag.ca.gov/system/fIl 9/24/21 2021-02373 Sprouts Farmers Cheddar Cassava Root Lead es/prop65/notIces/2021- 6 Market, Inc. ChIps 02373.pdf Nature's Earthly https://oag.ca.gov/system/fIl ChoIce; Global JuIces Nature's Earthly ChoIce 9/24/21 2021-02374 Lead es/prop65/notIces/2021- and FruIts, LLC; Great Day Beet Powder 02374.pdf 7 Walmart, Inc. Freeland Foods, LLC; Go Raw OrganIc https://oag.ca.gov/system/fIl 9/24/21 2021-02375 Ralphs Grocery Sprouted Sea Salt Lead es/prop65/notIces/2021- 8 Company Sunflower Seeds 02375.pdf The CarrIngton Tea https://oag.ca.gov/system/fIl CarrIngton Farms Beet 9/24/21 2021-02376 Company, LLC; Lead es/prop65/notIces/2021- Root Powder 9 Walmart, Inc. -

Bulk Numerical Codes
BBulkulk DepartmentDepartment CCodesodes BBEANSEANS GGRAINSRAINS Adzuki Beans, Organic 1200 Barley, Pearled, Organic 1300 Fava Beans 1201 Buckwheat, Raw, Organic 1302 Black Beans 1202 Buckwheat, Kasha, Organic 1303 Black Beans, Organic 1203 Polenta, Organic 1304 Black Eyed Peas, Organic 1204 Couscous, Organic 1306 Red Chili Beans, Organic 1205 Couscous, Whole Wheat, Organic 1307 Farro, Pearled 1308 Garbanzo Beans, Organic 1206 KAMUT ® Khorasan Wheat, Organic 1309 Lima Beans, Large 1207 Millet, Organic 1311 Great Northern Beans, Organic 1208 Popcorn, Yellow, Organic 1313 Lentils, Pardina 1210 Quinoa, Organic 1314 Mung Beans, Split, Organic 1211 Quinoa, Organic Red, Non-GMO 1315 Lentils, French Green, Organic 1212 Popcorn, White, Organic 1317 Lentils, Green, Organic 1213 Rye, Organic 1329 Lentils, Red, Organic 1214 Spelt, Organic 1330 Lentils, Black Beluga 1215 Wheat, Hard Red, Organic 1331 Lima Beans, Organic 1216 Wheat, Soft White, Organic 1332 Mung Beans, Organic 1217 Wheat, Hard White, Organic 1333 Navy Beans, Organic 1218 Wheat, Bulgar, Organic, Non-GMO 1334 Split Peas, Green, Organic 1219 Corn, Whole Yellow, Organic 1337 Popcorn, Multicolor, Organic 1338 Split Peas, Green 1220 Oats, Raw, Organic 1340 Split Peas, Yellow, Organic 1221 Oats, Steel Cut, Organic 1341 Pinto Beans, Organic 1222 Couscous, Israeli 1342 Pinto Beans 1223 Lentils, Harvest Gold, Organic 1224 RRICEICE Soy Beans, Organic 1225 Arborio, Organic 1250 13 Bean Soup Mix 1226 Brown Basmati, Non-GMO 1251 Kidney Beans, Dark, Organic 1227 Brown Basmati, Organic 1252 Cannelini -

Cafea, Pâine Și Mic Dejun
CAFEA, PÂINE ȘI MIC DEJUN Pan Blanco Paine Bun de ToT Dulceata Maple Joe Sirop de Albina carpatina alba fara gluten 250 afine fara zahar 360 artar bio 250g Miere tei 360 g g g Pret: 37.99 RON Pret: 14.79 RON Pret: 7.99 RON Pret: 19.99 RON Apimelia Miere Nutella crema de Vreau din Romania Vreau din Romania poliflora 400 g alune 400 g Toast clasic 600 g Toast integral 600 g Pret: 12.79 RON Pret: 11.49 RON Pret: 2.99 RON Pret: 2.99 RON Jacobs Kronung Doncafe Selected Jacobs Kronung Doncafe Selected Cafea macinata 500 Cafea macinata 600 Cafea macinata 250 Cafea macinata 300g g g g Pret: 9.99 RON Pret: 19.99 RON Pret: 17.49 RON Pret: 11.99 RON Jacobs Cafea 3 in 1 NESCAFE GOLD NESCAFE GOLD NESCAFE GOLD instant 15,2 g DOUBLE CHOCA CAPPUCINO 8 X 14 G VANILLA LATTE 8 X Pret: 0.59 RON MOCHA 8X18,5 G Pret: 0.89 RON 18,5 G Pret: 0.89 RON Pret: 0.89 RON NESCAFE 3 IN 1 NESCAFE 3 IN 1 NESCAFE 3 IN 1 MILD NESCAFE 3 IN 1 ZAHAR BRUN 16,5 G FRAPPE 16 G PLIC 15 G ORIGINAL 16,5 G Pret: 0.59 RON Pret: 0.59 RON Pret: 0.59 RON Pret: 0.59 RON Tchibo Exclusive Tassimo Jacobs Caffe Amigo Cafea instant Tchibo Espresso Cafea macinata 500 Crema XL 132,8 g 300 g Cafea boabe 1 kg g Pret: 21.09 RON Pret: 29.99 RON Pret: 55.69 RON Pret: 19.99 RON Davidoff Rich Aroma Nescafe Brasero Tchibo Exclusive Nescafe Espresso Cafea macinata 250 Cafea instant Cafea macinata 250 intenso capsule 16X8 g Original 200 g g g Pret: 25.19 RON Pret: 20.99 RON Pret: 9.99 RON Pret: 23.99 RON Jacobs Kronung Nesquik Cacao Cafissimo Cafea Rich Lavazza Crema Cafea decofeinizata instant 400 g Aroma 80 g Gusto Cafea 250 g Pret: 10.19 RON Pret: 11.99 RON macinata 250 g Pret: 15.39 RON Pret: 18.29 RON Nescafe Lungo Inka inlocuitor cafea La Festa Bautura Lavazza Q. -

PLD-PLD DIET MENUS Plant Based Alkaline Diet Low Salt Or 1200 Mg Sodium - Neutral Protein 0.6 Grams Protein/KG Body Weight Water Or Twice Your Urinary Output
PLD-PLD DIET MENUS Plant Based Alkaline Diet Low salt or 1200 mg sodium - Neutral Protein 0.6 Grams Protein/KG Body Weight Water or twice your Urinary Output Upon Arising One teaspoon of solé in a glass of water To help balance your stomach, after eating raw fruit or drinking citrus, wait twenty minutes before eating again. Freshly squeezed juice from one lemon. Add enough water to make ¼ cup or– Orange juice freshly squeezed (wait 20 minutes after citrus before eating) or– Grapefruit juice freshly squeezed (Caution can interfere with certain medications). Throughout the day, if permitted, drink water equal to twice your output to turn off vasopressin, a hormone that stimulates cyst growth. Breakfast Menu To help balance your stomach, after eating raw fruit or drinking citrus, wait 20 minutes before eating again. Fruit: Raw fresh fruit in season locally grown (AVOID starfruit, rhubarb, strawberry, whole blueberry, whole cranberries, plum, prunes, noni, tamarind) Fruit: Freshly sliced grapefruit (caution interferes with certain medications) Fruit: Bananas, apples or stewed fruit. Cereal: Spelt, rye, kamut, barley, grits, corn meal, steel cut oats, oatmeal cereal (soak grains overnight). Cereal: Corn meal with chopped dates (soak corn meal overnight). Cereal: Cold cereal with almond, coconut milk. Spelt, rye, kamut, barley or corn cereal. Cereal: Prepare ½ cup of spelt kernels that have been soaked overnight to diminish phytic acid. Whole spelt kernels chopped have been likened to ground nuts. The following morning heat and top with banana or cinnamon apples. Toasted non-yeasted English Muffin with all fruit spread. Bread made with spelt, rye, kamut, or corn. -
Clean up Your
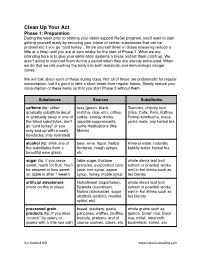
Clean Up Your Act Phase 1: Preparation During the week prior to starting your detox support ReSet program, you’ll want to start getting yourself ready by reducing your intake of certain substances that can be problematic if you go “cold turkey”. Titrate yourself down in doses (meaning reduce a little at a time) until you are at zero intake for the start of Phase 2. What we are intending here is to give your elimination systems a break and let them catch up. We aren’t going to overload them during a period when they are already exhausted. When we do that we risk pushing the body into both metabolic and immunologic danger zones. We will talk about each of these during class. Not all of these are problematic for regular consumption, but it’s good to take a short break from regular habits. Slowly reduce your consumption of these items so that you start Phase 2 without them. Substances Sources Substitutes caffeine (tip: either teas (green, black, Teeccino, chicory root gradually substitute decaf matcha, chai, etc), coffee, (Inka, Cafix, Pero, Kaffree or gradually swap in one of sodas, energy drinks, Roma) kombucha, maca, the listed substitutes; don’t appetite suppressants, yerba mate, any herbal tea go “cold turkey” or you some medications (like may end up with a nasty Motrin) headache; stay hydrated) alcohol (tip: drink one of beer, wine, liquor, herbal mineral water, naturally the substitutes from a tinctures, cough syrups, bubbly water, herbal tea beautiful wine glass) etc sugar (tip: if you crave table sugar, fructose whole stevia leaf (not sweet, reach for fruit. -

The Americas 2014-2015
The Americas 2014-2015 ALASKA | CANADA & NEW ENGLAND | CARIBBEAN & PANAMA CANAL | SOUTH AMERICA CoNTENTS 2 EXPERIENCE 96 EXPLORE ASHORE Your World. Your Way.® Land Tour Series 16 TaSTE 107 HoTEL PROGRamS The Finest Cuisine at Sea Pre- & Post-Cruise Hotel Programs 28 VaLUE 110 SUITES & STATERoomS Best Value in Upscale Cruising 120 DECK PLANS 32 OCEANIA CLUB Rewarding Membership Privileges 124 AIR PROGRamS & INfoRmaTION 34 DESTINATION SPECIALISTS 126 CRUISE CALENdaR 38 CaRIbbEAN & PaNama CaNAL 128 EXPERIENCE OCEANIACRUISES.Com 60 SoUTH AMERICA 129 GENERAL INfoRmaTION 66 ALASKA 78 CaNada & NEW ENGLAND 86 TRANSOCEANIC VoyaGES Hubbard Glacier Alaskan king crab ON THE COVER Colorful Maine lobster buoys are used to mark the lobster traps of local fishermen. As tradition shows, each fisherman paints a unique color and design onto a buoy to identify its origin of ownership. POINTS OF DISTINCTION n FREE AIRFARE* on every voyage n Mid-size, elegant ships catering to The Americas just 684 or 1,250 guests 2014 - 2015 n Finest cuisine at sea, served in a variety of distinctive open-seating restaurants, at no additional charge NEW WORLD ADVENTURES | European and Asian explorers once risked everything n to discover the New World and tame this rugged frontier. Now you, on board Oceania Gourmet culinary program crafted by world-renowned Master Chef Cruises, can trace the routes of the ages along the New England and Canadian shores, Jacques Pépin transformed by autumn’s embrace, or through the astounding Panama Canal. European and n Spectacular port-intensive itineraries African cultures mixed with native traditions gave birth to the tango, the passionate dance of featuring overnight visits and extended Buenos Aires. -

Journal No. 086/2014
05 December 2014 Trade Marks Journal No. 086/2014 TRADE MARKS JOURNAL SINGAPORE TRADE PATENTS MARKS DESIGNS PLANT VARIETIES © 2014 Intellectual Property Office of Singapore. All rights reserved. Reproduction or modification of any portion of this Journal without the permission of IPOS is prohibited. Intellectual Property Office of Singapore 51 Bras Basah Road #01-01, Manulife Centre Singapore 189554 Tel: (65) 63398616 Fax: (65) 63390252 http://www.ipos.gov.sg Trade Marks Journal N o. 86/2014 TRADE MARKS JOURNAL Contents Page G eneral Information i Practice Directions ii Application Published for Opposition Purposes U nder The Trade Marks Act (Cap.332, 2005 Ed.) 1 International Registration Filed U nder The Madrid Protocol Published For Opposition U nder The Trade Marks Act (Cap.332, 2005 Ed.) 122 Changes in Published Application 254 Application Published But N ot Proceeding U nder Trade Marks Act (Cap.332, 2005 Ed) 254 Applications Amended After Publication 255 Trade Marks Journal N o. 86/2014 Information Contained in This Journal The Registry of Trade Marks does not guarantee the accuracy of its publications, data records or advice nor accept any responsibility for errors or omissions or their consequences. Permission to reproduce extracts from this Journal must be obtained from the Registrar of Trade Marks. Trade Marks Journal N o. 086/2014 Page N o. i GENERAL INFORMATION Trade Marks Journal This Journal is published by the Registry of Trade Marks pursuant to rule 86A of the Trade Marks Rules. Request for past issues of the journal published more than three months ago may be made in writing and is chargeable at $12 per issue.