6-Oxo) and Metabolites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CAS Number Index
2334 CAS Number Index CAS # Page Name CAS # Page Name CAS # Page Name 50-00-0 905 Formaldehyde 56-81-5 967 Glycerol 61-90-5 1135 Leucine 50-02-2 596 Dexamethasone 56-85-9 963 Glutamine 62-44-2 1640 Phenacetin 50-06-6 1654 Phenobarbital 57-00-1 514 Creatine 62-46-4 1166 α-Lipoic acid 50-11-3 1288 Metharbital 57-22-7 2229 Vincristine 62-53-3 131 Aniline 50-12-4 1245 Mephenytoin 57-24-9 1950 Strychnine 62-73-7 626 Dichlorvos 50-23-7 1017 Hydrocortisone 57-27-2 1428 Morphine 63-05-8 127 Androstenedione 50-24-8 1739 Prednisolone 57-41-0 1672 Phenytoin 63-25-2 335 Carbaryl 50-29-3 569 DDT 57-42-1 1239 Meperidine 63-75-2 142 Arecoline 50-33-9 1666 Phenylbutazone 57-43-2 108 Amobarbital 64-04-0 1648 Phenethylamine 50-34-0 1770 Propantheline bromide 57-44-3 191 Barbital 64-13-1 1308 p-Methoxyamphetamine 50-35-1 2054 Thalidomide 57-47-6 1683 Physostigmine 64-17-5 784 Ethanol 50-36-2 497 Cocaine 57-53-4 1249 Meprobamate 64-18-6 909 Formic acid 50-37-3 1197 Lysergic acid diethylamide 57-55-6 1782 Propylene glycol 64-77-7 2104 Tolbutamide 50-44-2 1253 6-Mercaptopurine 57-66-9 1751 Probenecid 64-86-8 506 Colchicine 50-47-5 589 Desipramine 57-74-9 398 Chlordane 65-23-6 1802 Pyridoxine 50-48-6 103 Amitriptyline 57-92-1 1947 Streptomycin 65-29-2 931 Gallamine 50-49-7 1053 Imipramine 57-94-3 2179 Tubocurarine chloride 65-45-2 1888 Salicylamide 50-52-2 2071 Thioridazine 57-96-5 1966 Sulfinpyrazone 65-49-6 98 p-Aminosalicylic acid 50-53-3 426 Chlorpromazine 58-00-4 138 Apomorphine 66-76-2 632 Dicumarol 50-55-5 1841 Reserpine 58-05-9 1136 Leucovorin 66-79-5 -

Precursor Ion Scanning for the Detection of New Steroid Markers
“Precursor ion scanning for the detection of new steroid markers. Routine application for the open screening of anabolic steroids and evaluation of population factors in the detectability of these markers” P. Mendoza, F. Delbeke, K. Deventer, P. Meuleman, J. Segura, R. Ventura (Fondacio IMIM, Barcelona, Spain) K. Deventer, P. Van Eenoo, O. Pozo Mendoza (Ghent University, Belgium) PROJECT REVIEW This project aims at four goals: A. Interpretation and evaluation of precursor ion scanning chromatograms (urine profile recognition) In the field of anti-doping control, chromatograms from target steroid-analysis are generally evaluated and interpreted visually by the analyst which is trained for this purpose. A similar approach will be adopted for evaluating the precursor scanning chromatograms. To get acquainted with the chromatograms, around 50 blank samples will be analysed. In a second step precursor ion scan chromatograms obtained from controlled administration studies (e.g. methyltestosterone, methanedienone,…) and from adverse analytical findings from routine target screening will also be investigated to get acquainted with positive samples. The applicability of instrument software for this purpose will be evaluated. B. Application to real samples Urine-samples will be analysed. These samples will include all out of competition samples and both out of competition samples and in competition samples C. Study of the influence of different population factors in the detectability of different markers for several anabolic steroids A single dose of the previously studied steroids (stanozolol and methyltestosterone) will be administered to six volunteers belonging to different population groups. Urine will be collected for three weeks and a method including all feasible markers will be applied. -

Dalton Pharma Catalogue Steroids 12-19-12
2012/2013 Version: December 31, 2012 Steroid Analytical Reference Standards and Drug Impurities Dalton Pharma Services: A Health Canada Approved Facility Level-8 Controlled Substances License on-site SCC Approved GLP Facility North American Manufactured and Shipped To Order Call: 1.800.567.5060 To Order Call: 416.661.2102 Email: [email protected] Email: [email protected] Page 2 Dalton Standards and Impurities Table of Contents Introduction/Company Profile 4 Custom Synthesis Overview 6 Contract Research/Medicinal Chemistry 7 Process Development Overview 8 Analytical Services 9 GMP API & Sterile Filling Services 11 Formulation Development Overview 12 Liposomes 13 Dalton Standards and Impurities 14 Establishment License 16 Certificate example 17 Steroids 18 Page 3 To Order Call: 1.800.567.5060 To Order Email: [email protected] Call: Company Profile About Dalton Pharma Services Dalton Pharma Services is a privately-held pharmaceutical services company that has been producing Fine Chemi- cals on a custom basis for research and chemical supply houses for over twenty-five years (Fluka, Aldrich, Sigma, Acros). We have identified and supplied many new re- agents for biological and pharmaceutical applications, this includes novel analogues and impurities of active pharma- ceuticals ingredients, as well as new linkers. We are lead- ers in process development, process improvement, and in the field of isolation of kilo quantities of biologically active molecules from natural sources. Dalton excels at advancing projects from R&D into GMP environments for its customers. With the ability to manufacture cGMP API's from bench scales to multi kilos we can meet most clinical and small scale commercial requirements. -

Boldione Metabolismo
CORE Metadata, citation and similar papers at core.ac.uk Provided by Ghent University Academic Bibliography Detection and characterization of urinary metabolites of boldione. Part I: phase I metabolites excreted free and conjugated with glucuronic acid or sulphate and released after alkaline treatment of the urine. C. Gómez1,2, O.J. Pozo1, , A. Fabregat1,2, , J. Marcos1,2, K. Deventer3, P. van Eenoo3, J. Segura1,2, R. Ventura1,2 1 Bioanalysis Research Group, IMIM, Hospital del Mar, Doctor Aiguader 88, 08003 Barcelona, Spain 2 Department of Experimental and Health Sciences, Universitat Pompeu Fabra, Doctor Aiguader 88, 08003 Barcelona, Spain 3 DoCoLab, Univeristy of Ghent, Technologiepark 30, B-9052, Zwijnaarde, Belgium Corresponding author: Rosa Ventura Bioanalysis and Analytical Services Research Group IMIM, Institut de Recerca Hospital del Mar Doctor Aiguader, 88 08003 Barcelona, Spain Tel. 34 93 3160471 Fax 34 93 3160499 E-mail: [email protected] 1 Abstract Boldione (1,4-androstadiene-3,17-dione) is included in the list of prohibited substances, issued by the World Antidoping Agency, in the group of exogenous anabolic steroids. Endogenous production of low concentrations of boldione has also been reported. The objective of the study was to assess boldione metabolism in humans. Detection of boldione metabolites was accomplished by analysis by liquid chromatography coupled to tandem mass spectrometry of urine samples obtained after administration of the drug and subjected to different sample preparation procedures to analyze the different metabolic fractions. In addition to boldione, eight metabolites were detected in the free fraction. Three of them were identified by comparison with standards: androsta-1,4,6- triene-3,17-dione (M5), (5α)-1-androstenedione (M6)- and (5α)-1-testosterone (M8). -

Pros and Cons Controversy on Molecular Imaging and Dynamic
Open Access Archives of Biotechnology and Biomedicine Research Article Pros and Cons Controversy on Molecular Imaging and Dynamics of Double- ISSN Standard DNA/RNA of Human Preserving 2639-6777 Stem Cells-Binding Nano Molecules with Androgens/Anabolic Steroids (AAS) or Testosterone Derivatives through Tracking of Helium-4 Nucleus (Alpha Particle) Using Synchrotron Radiation Alireza Heidari* Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, USA *Address for Correspondence: Dr. Alireza Abstract Heidari, Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, In the current study, we have investigated pros and cons controversy on molecular imaging and dynamics USA, Email: of double-standard DNA/RNA of human preserving stem cells-binding Nano molecules with Androgens/ [email protected]; Anabolic Steroids (AAS) or Testosterone derivatives through tracking of Helium-4 nucleus (Alpha particle) using [email protected] synchrotron radiation. In this regard, the enzymatic oxidation of double-standard DNA/RNA of human preserving Submitted: 31 October 2017 stem cells-binding Nano molecules by haem peroxidases (or heme peroxidases) such as Horseradish Peroxidase Approved: 13 November 2017 (HPR), Chloroperoxidase (CPO), Lactoperoxidase (LPO) and Lignin Peroxidase (LiP) is an important process from Published: 15 November 2017 both the synthetic and mechanistic point of view. Copyright: 2017 Heidari A. This is an open access article distributed under the Creative -

Plants Are Capable of Synthesizing Animal Steroid Hormones
molecules Review Plants are Capable of Synthesizing Animal Steroid Hormones Danuše Tarkowská Laboratory of Growth Regulators, Centre of the Region Haná for Biotechnological and Agricultural Research, Institute of Experimental Botany, Czech Academy of Sciences, and Faculty of Science, Palacký University, CZ-783 71 Olomouc, Czech Republic; [email protected]; Tel.: +420-585-631-478 Received: 27 June 2019; Accepted: 15 July 2019; Published: 16 July 2019 Abstract: As a result of the findings of scientists working on the biosynthesis and metabolism of steroids in the plant and animal kingdoms over the past five decades, it has become apparent that those compounds that naturally occur in animals can also be found as natural constituents of plants and vice versa, i.e., they have essentially the same fate in the majority of living organisms. This review summarizes the current state of knowledge on the occurrence of animal steroid hormones in the plant kingdom, particularly focusing on progesterone, testosterone, androstadienedione (boldione), androstenedione, and estrogens. Keywords: natural sterols; plants; animals; steroid hormones; estrogens; progesterone; testosterone; boldenone; boldione; androstenedione 1. Introduction The plant and animal kingdoms are not two completely separate worlds coexisting on this planet, but, on the contrary, they are two worlds whose evolution has taken place simultaneously, hand in hand with each other. It is therefore more than obvious that some substances being synthesized in nature for a particular purpose can occur in both plant and animal organisms. Certain compounds that regulate growth and development in plants may also control cellular growth and differentiation processes in animals, and vice versa. An example of such compounds may be sterols, i.e., steroid alcohols. -

Legally Defensible Steroid Testing Services
LGC in drug and alcohol testing Legally defensible steroid testing services Anabolic steroid misuse has Anabolic steroids, technically known as anabolic- historically been associated with sport androgenic steroids (AAS), are drugs that are structurally related to the naturally produced and doping scandals over the past steroid testosterone. They increase protein within 25 years. More recently, due in part cells, especially in skeletal muscles. Anabolic to their ready availability over the steroids also have androgenic and virilising internet both as steroid preparations properties, including the development and and as constituents of steroid based maintenance of masculine characteristics such as the growth of the vocal cords, testicles (primary nutritional supplements, the misuse of sexual characteristics) and body hair (secondary anabolic steroids has spread to wider sexual characteristics). areas of society including regular gym users and sub-elite sportspeople. www.lgcgroup.com/steroids • [email protected] Science for a safer world Brazil • Bulgaria • China • Czech Republic • France • Germany • Hungary • India • Ireland • Italy • Netherlands Poland • Romania • Russia • Scandanavia • South Africa • Spain • Turkey • United Kingdom • USA No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording or any retrieval system, without the written permission of the copyright holder. © LGC Limited, 2014. All rights reserved. 3995/LB/0814 Anabolic steroids were first synthesized in the and for cause workplace screen for anabolic Bolasterone 1930s, and are now used therapeutically in steroids. This premier service is comparable to that Boldenone medicine to stimulate bone growth and appetite, currently provided by WADA accredited laboratories Boldione Calusterone induce male puberty and treat chronic wasting supporting doping control programmes in sport. -

The Modulatory Role of Vitamin C in Boldenone Undecylenate Induced Testicular Oxidative Damage and Androgen Receptor Dysregulation in Adult Male Rats
antioxidants Article The Modulatory Role of Vitamin C in Boldenone Undecylenate Induced Testicular Oxidative Damage and Androgen Receptor Dysregulation in Adult Male Rats Amany Behairy 1 , Nabela I. El-Sharkawy 2, Taghred M. Saber 2, Mohamed Mohamed Soliman 3,4 , Mohamed M.M. Metwally 5 , Ghada I. Abd El-Rahman 6, Yasmina M. Abd-Elhakim 2,* and Maha M. El Deib 7 1 Department of Physiology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511, Egypt; [email protected] 2 Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511, Egypt; [email protected] (N.I.E.-S.); [email protected] (T.M.S.) 3 Clinical Laboratory Sciences Department, Turabah University College, Taif University, P.O. Box 11099, Taif 21944, Saudi Arabia; [email protected] 4 Biochemistry Department, Faculty of Veterinary Medicine, Benha University, Benha 13736, Egypt 5 Department of Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511, Egypt; [email protected] 6 Department of Clinical Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511, Egypt; [email protected] 7 Department of Biochemistry, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511,Egypt; [email protected] * Correspondence: [email protected] Received: 11 October 2020; Accepted: 26 October 2020; Published: 28 October 2020 Abstract: Background: This study explored the effect of vitamin C (Vit-C) administration on the reproductive function of adult male Wistar rats injected with boldenone undecylenate (BOL). Methods: Rats were randomly assigned into control, vehicle control, Vit-C (120 mg/kg b.wt./day, orally), BOL (received 5 mg/kg b.wt./week, IM) and BOL+Vit-C-treated groups. -

90-91. Schedule III Controlled Substances
§ 90-91. Schedule III controlled substances. This schedule includes the controlled substances listed or to be listed by whatever official name, common or usual name, chemical name, or trade name designated. In determining that a substance comes within this schedule, the Commission shall find: a potential for abuse less than the substances listed in Schedules I and II; currently accepted medical use in the United States; and abuse may lead to moderate or low physical dependence or high psychological dependence. The following controlled substances are included in this schedule: (a) Repealed by Session Laws 1973, c. 540, s. 5. (b) Any material, compound, mixture, or preparation which contains any quantity of the following substances having a depressant effect on the central nervous system unless specifically exempted or listed in another schedule: 1. Any substance which contains any quantity of a derivative of barbituric acid, or any salt of a derivative of barbituric acid. 2. Chlorhexadol. 3. Repealed by Session Laws 1993, c. 319, s. 5. 4. Lysergic acid. 5. Lysergic acid amide. 6. Methyprylon. 7. Sulfondiethylmethane. 8. Sulfonethylmethane. 9. Sulfonmethane. 9a. Tiletamine and zolazepam or any salt thereof. Some trade or other names for tiletamine-zolazepam combination product: Telazol. Some trade or other names for tiletamine: 2-(ethylamino)-2-(2-thienyl)-cyclohexanone. Some trade or other names for zolazepam: 4-(2-fluorophenyl)-6,8-dihydro-1,3,8-trimethylpyrazolo-[3,4-e][1,4]/y-diaze pin-7(1H)-one. flupyrazapon. 10. Any compound, mixture or preparation containing (i) Amobarbital. (ii) Secobarbital. (iii) Pentobarbital. or any salt thereof and one or more active ingredients which are not included in any other schedule. -

Drug/Substance Trade Name(S)
A B C D E F G H I J K 1 Drug/Substance Trade Name(s) Drug Class Existing Penalty Class Special Notation T1:Doping/Endangerment Level T2: Mismanagement Level Comments Methylenedioxypyrovalerone is a stimulant of the cathinone class which acts as a 3,4-methylenedioxypyprovaleroneMDPV, “bath salts” norepinephrine-dopamine reuptake inhibitor. It was first developed in the 1960s by a team at 1 A Yes A A 2 Boehringer Ingelheim. No 3 Alfentanil Alfenta Narcotic used to control pain and keep patients asleep during surgery. 1 A Yes A No A Aminoxafen, Aminorex is a weight loss stimulant drug. It was withdrawn from the market after it was found Aminorex Aminoxaphen, Apiquel, to cause pulmonary hypertension. 1 A Yes A A 4 McN-742, Menocil No Amphetamine is a potent central nervous system stimulant that is used in the treatment of Amphetamine Speed, Upper 1 A Yes A A 5 attention deficit hyperactivity disorder, narcolepsy, and obesity. No Anileridine is a synthetic analgesic drug and is a member of the piperidine class of analgesic Anileridine Leritine 1 A Yes A A 6 agents developed by Merck & Co. in the 1950s. No Dopamine promoter used to treat loss of muscle movement control caused by Parkinson's Apomorphine Apokyn, Ixense 1 A Yes A A 7 disease. No Recreational drug with euphoriant and stimulant properties. The effects produced by BZP are comparable to those produced by amphetamine. It is often claimed that BZP was originally Benzylpiperazine BZP 1 A Yes A A synthesized as a potential antihelminthic (anti-parasitic) agent for use in farm animals. -

Anti-Doping Convention
European Treaty Series - No. 135 Anti-Doping Convention Strasbourg, 16.XI.1989 Appendix – AMENDMENTS TO THE APPENDIX (approved by the Monitoring Group under Article 11.1.b of the Convention at its 51st meeting) (Strasbourg, 24 October 2019) THE 2020 PROHIBITED LIST - WORLD ANTI-DOPING CODE DATE OF ENTRY INTO FORCE: 1 JANUARY 2020 SUBSTANCES AND METHODS PROHIBITED AT ALL TIMES (IN- AND OUT-OF-COMPETITION) IN ACCORDANCE WITH ARTICLE 4.2.2 OF THE WORLD ANTI-DOPING CODE, ALL PROHIBITED SUBSTANCES SHALL BE CONSIDERED AS “SPECIFIED SUBSTANCES” EXCEPT SUBSTANCES IN CLASSES S1, S2, S.4.4, S.4.5, S6.A, AND PROHIBITED METHODS M1, M2 AND M3. PROHIBITED SUBSTANCES 1-Testosterone (17β-hydroxy-5α-androst-1- en-3-one); S0. NON-APPROVED SUBSTANCES 4-Androstenediol (androst-4-ene-3β,17β-diol); 4-Hydroxytestosterone (4,17β- Any pharmacological substance which is not dihydroxyandrost-4-en-3one); addressed by any of the subsequent sections of 5-Androstenedione (androst-5-ene-3,17- the List and with no current approval by any dione); governmental regulatory health authority for 7α-hydroxy-DHEA; human therapeutic use (e.g. drugs under pre- 7β-hydroxy-DHEA; clinical or clinical development or discontinued, 7-Keto-DHEA; designer drugs, substances approved only for 19-Norandrostenediol (estr-4-ene-3,17-diol); veterinary use) is prohibited at all times. 19-Norandrostenedione (estr-4-ene-3,17- dione); S1. ANABOLIC AGENTS Androstanolone (5α-dihydrotestosterone, 17β- hydroxy-5αandrostan-3-one); Anabolic agents are prohibited. Androstenediol (androst-5-ene-3β,17β-diol); -
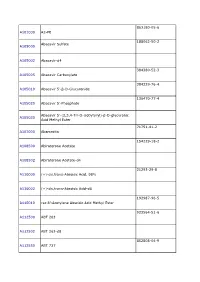
TRC Price List 2010
863180-05-6 A101000 A2-PE 188062-50-2 Abacavir Sulfate A105000 A105002 Abacavir-d4 384380-52-3 A105005 Abacavir Carboxylate 384329-76-4 A105010 Abacavir 5’-β-D-Glucuronide 136470-77-4 A105020 Abacavir 5’-Phosphate Abacavir 5’-(2,3,4-Tri-O-isobytyryl)-β-D-glucuronic A105030 Acid Methyl Ester 71751-41-2 A107000 Abamectin 154229-18-2 A108500 Abiraterone Acetate A108502 Abiraterone Acetate-d4 21293-29-8 A110000 (+)-cis,trans-Abscisic Acid, 98% A110002 (+)-cis,trans-Abscisic Acid-d6 192987-96-5 A110010 rac 8’-Acetylene Abscisic Acid Methyl Ester 923564-51-6 A112500 ABT 263 A112502 ABT 263-d8 852808-04-9 A112550 ABT 737 A112552 ABT 737-d8 912444-00-9 A112580 ABT 888 447407-36-5 A115600 AC 42 77337-73-6 A120000 Acamprosate Calcium A120002 Acamprosate-d12 Calcium Trihydrate A120003 Acamprosate-d6 Calcium Trihydrate 56180-94-0 A123500 Acarbose A123502 Acarbose-d4 117065-98-2 A123505 Acarbose Tridecaacetate 34381-68-5 A123800 Acebutolol Hydrochloride Acebutolol 37517-30-9 A123801 Discontinued.