Ecological Effects of Ditching and Ditch-Plugging in New England Salt Marshes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Preliminary Flood Insurance Study
VOLUME 4 OF 4 YORK COUNTY, MAINE (ALL JURISDICTIONS) COMMUNITY NAME NUMBER COMMUNITY NAME NUMBER ACTON, TOWN OF 230190 OGUNQUIT, TOWN OF 230632 ALFRED, TOWN OF 230191 OLD ORCHARD BEACH, TOWN OF 230153 ARUNDEL, TOWN Of 230192 PARSONSFIELD, TOWN OF 230154 BERWICK, TOWN OF 230144 SACO, CITY OF 230155 BIDDEFORD, CITY OF 230145 SANFORD, CITY OF 230156 BUXTON, TOWN OF 230146 SHAPLEIGH, TOWN OF 230198 CORNISH, TOWN OF 230147 SOUTH BERWICK, TOWN OF 230157 DAYTON, TOWN OF 230148 WATERBORO, TOWN OF 230199 ELIOT, TOWN OF 230149 WELLS, TOWN OF 230158 HOLLIS, TOWN OF 230150 YORK, TOWN OF 230159 KENNEBUNK, TOWN OF 230151 KENNEBUNKPORT, TOWN OF 230170 KITTERY, TOWN OF 230171 LEBANON, TOWN OF 230193 LIMERICK, TOWN OF 230194 LIMINGTON, TOWN OF 230152 LYMAN, TOWN OF 230195 NEWFIELD, TOWN OF 230196 NORTH BERWICK, TOWN OF 230197 EFFECTIVE: FLOOD INSURANCE STUDY NUMBER 23005CV004A Version Number 2.3.2.1 TABLE OF CONTENTS Volume 1 Page SECTION 1.0 – INTRODUCTION 1 1.1 The National Flood Insurance Program 1 1.2 Purpose of this Flood Insurance Study Report 2 1.3 Jurisdictions Included in the Flood Insurance Study Project 2 1.4 Considerations for using this Flood Insurance Study Report 20 SECTION 2.0 – FLOODPLAIN MANAGEMENT APPLICATIONS 31 2.1 Floodplain Boundaries 31 2.2 Floodways 43 2.3 Base Flood Elevations 44 2.4 Non-Encroachment Zones 44 2.5 Coastal Flood Hazard Areas 45 2.5.1 Water Elevations and the Effects of Waves 45 2.5.2 Floodplain Boundaries and BFEs for Coastal Areas 46 2.5.3 Coastal High Hazard Areas 47 2.5.4 Limit of Moderate Wave Action 48 SECTION -

Implementation of the Great Works River Non-Point Source Pollution Watershed Management Plan" (2010)
University of New Hampshire University of New Hampshire Scholars' Repository PREP Publications Piscataqua Region Estuaries Partnership 2010 Implementation of the Great Works River Non- Point Source Pollution Watershed Management Plan PREP Follow this and additional works at: http://scholars.unh.edu/prep Part of the Marine Biology Commons Recommended Citation PREP, "Implementation of the Great Works River Non-Point Source Pollution Watershed Management Plan" (2010). PREP Publications. Paper 72. http://scholars.unh.edu/prep/72 This Article is brought to you for free and open access by the Piscataqua Region Estuaries Partnership at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in PREP Publications by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. Implementation of the Great Works River Non- point Source Pollution Watershed Management Plan 09-060 A Final Report to The Piscataqua Region Estuaries Partnership Submitted by Tin Smith Wells National Estuarine Research Reserve 342 Laudholm Farm Road Wells Maine 04090 207-646-1555 x 119 [email protected] May 2010 This project was funded in part by a grant from the Piscataqua Region Estuaries Partnership as authorized by the U.S. Environmental Protection Agency’s National Estuary Program. Table of Contents I. Abstract 1 II. Executive Summary 1 III. Introduction 3 IV. Project Goals and Objectives 4 V. Activities 5 VI. Outcomes 7 VII. Appendices 9 I. Abstract This project was a collaboration between the Wells National Estuarine Research Reserve, Great Works Regional Land Trust, and the Great Works River Watershed Coalition to implement five of the “Highest Priority” tasks from the Action Plan of the Great Works River Nonpoint Source Pollution Watershed Management Plan (2007). -

Status of Conservation Planning for Watersheds of Southern Maine
watermark volume 26 issue 1 5 stewardship / conserving natural resources Status of Conservation Planning for Watersheds of Southern Maine The Wells Reserve has produced or assisted with every key conservation planning document prepared for What is a watershed? southern Maine watersheds over the past decade. Use this chart to learn which plans cover your town, then An area in which water, sediments, download the plan(s) from our website or read them at the Dorothy Fish Coastal Resource Library. and dissolved material drain to a To get involved in land or water protection in your area, contact the individuals or organizations named common outlet, such as a river, lake, in the plans. Key contacts for most towns and watersheds can also be found at swim.wellsreserve.org. If these bay, or ocean. avenues don’t work out, call the Wells Reserve stewardship coordinator, Tin Smith, at 646-1555 ext 119. U.S. Environmental Protection Agency Watershed A geographic area in which water flows on its way to a larger water body, such as a stream, river, estuary, lake, or Town Bridges Swamp Neddick River Cape River Works Great Josias River Kennebunk River Little River Mousam River Ogunquit River River Piscataqua River Salmon Falls Southside Brook Creek Spruce River Webhannet River York ocean. Coastal and ocean resources Acton 7 11 are affected not only by activities in Alfred 4 7 coastal areas but also by those in Arundel 4 7 upland watersheds. Berwick 2,3 2,11 U.S. Commission on Ocean Policy Eliot 2,11 2,11 2,13,14 2,16,17 Kennebunk 4 5,6 7 Kennebunkport 4 Why these watersheds? Kittery 11 11 0 13,14 16,17 The chart shows the Maine watersheds Lebanon 11 and towns that are included in the web- Lyman 4 7 based Seacoast Watershed Information North Berwick 2,3 2,11 Manager (swim.wellsreserve.org). -

Kennebec Estuary Focus Areas of Statewide Ecological Significance Kennebec Estuary
Focus Areas of Statewide Ecological Significance: Kennebec Estuary Focus Areas of Statewide Ecological Significance Kennebec Estuary WHY IS THIS AREA SIGNIFICANT? The Kennebec Estuary Focus Area contains more than 20 percent of Maine’s tidal marshes, a significant percentage of Maine’s sandy beach and associated dune Biophysical Region habitats, and globally rare pitch pine • Central Maine Embayment woodland communities. More than two • Cacso Bay Coast dozen rare plant species inhabit the area’s diverse natural communities. Numerous imperiled species of animals have been documented in the Focus Area, and it contains some of the state’s best habitat for bald eagles. OPPORTUNITIES FOR CONSERVATION » Work with willing landowners to permanently protect remaining undeveloped areas. » Encourage town planners to improve approaches to development that may impact Focus Area functions. » Educate recreational users about the ecological and economic benefits provided by the Focus Area. » Monitor invasive plants to detect problems early. » Find ways to mitigate past and future contamination of the watershed. For more conservation opportunities, visit the Beginning with Habitat Online Toolbox: www.beginningwithhabitat.org/ toolbox/about_toolbox.html. Rare Animals Rare Plants Natural Communities Bald Eagle Lilaeopsis Estuary Bur-marigold Coastal Dune-marsh Ecosystem Spotted Turtle Mudwort Long-leaved Bluet Maritime Spruce–Fir Forest Harlequin Duck Dwarf Bulrush Estuary Monkeyflower Pitch Pine Dune Woodland Tidewater Mucket Marsh Bulrush Smooth Sandwort -
Firm Map Index Nfip
MAP NUMBER MAP DATES Great Works Goodall MAP REPOSITORIES River Brook TOWN OF LYMAN L i This FIRM Index displays the map date for each t B t Duck Brook (Maps available for reference only, not for l CITY OF SACO og e Estes Lake FIRM panel at the time that this Index was Br R distribution.) ook Mousam ive TOWN OF W TOWN OF printed. Because this Index may not be r River Mousam ard Brook 9 River ALFRED ARUNDEL (! distributed to unaffected communities in 23031C0361G 23031C0362G Bush Brook 23031C0391G 23031C0392G subsequent revisions, users may determine the 23031C0411G 23031C0412G 23031C0416G 23031C0417G CITY OF BIDDEFORD ACTON, TOWN OF: Salmon 23031C0440G 23031C0441G 23031C0442G 23031C0461G current map date for each FIRM panel by visiting TOWN OF (!35 23031C0462G 23031C0466G 23031C0467G 23031C0486G Town Hall Falls River Round Swamps the FEMA Map Service Center website at S 35 H Road KENNEBUNK mit Little Old Fishing k Brook h http://msc.fema.gov, or by calling the FEMA Map SANFORD Mousam o B River Acton, Maine 04001 23031C0370G Pond 1 o r r o 23031C0390G REGIONAL Kennebunk ¤£ ok Information eXchange (FMIX) at 1-877-336-2627. River B River l B Sand AIRPORT il a Great Works River tso ALFRED, TOWN OF: Pond M n 23031C0495G*** Communities annexing land on adjacent FIRM f R TOWN OF LEBANON i Town Hall 99 of v 23031C0490G*** (! e panels must obtain a current copy of the 4 G 23031C0363G 23031C0364G** (! TOWN OF r 16 Saco Road 23031C0393G adjacent panel as well as the current FIRM 23031C0394G 23031C0413G 23031C0420G KENNEBUNKPORT Alfred, Maine 04002 23031C0414G 23031C0438G 23031C0439G 23031C0443G 23031C0444G 23031C0463G Index. -
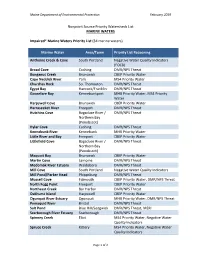
Nonpoint Source Priority Watersheds List MARINE WATERS
Maine Department of Environmental Protection February 2019 Nonpoint Source Priority Watersheds List MARINE WATERS Impaired* Marine Waters Priority List (34 marine waters) Marine Water Area/Town Priority List Reasoning Anthoine Creek & Cove South Portland Negative Water Quality Indicators (FOCB) Broad Cove Cushing DMR/NPS Threat Bunganuc Creek Brunswick CBEP Priority Water Cape Neddick River York MS4 Priority Water Churches Rock So. Thomaston DMR/NPS Threat Egypt Bay Hancock/Franklin DMR/NPS Threat Goosefare Bay Kennebunkport MHB Priority Water, MS4 Priority Water Harpswell Cove Brunswick CBEP Priority Water Harraseeket River Freeport DMR/NPS Threat Hutchins Cove Bagaduce River / DMR/NPS Threat Northern Bay (Penobscot) Hyler Cove Cushing DMR/NPS Threat Kennebunk River Kennebunk MHB Priority Water Little River and Bay Freeport CBEP Priority Water Littlefield Cove Bagaduce River / DMR/NPS Threat Northern Bay (Penobscot) Maquoit Bay Brunswick CBEP Priority Water Martin Cove Lamoine DMR/NPS Threat Medomak River Estuary Waldoboro DMR/NPS Threat Mill Cove South Portland Negative Water Quality Indicators Mill Pond/Parker Head Phippsburg DMR/NPS Threat Mussell Cove Falmouth CBEP Priority Water, DMR/NPS Threat North Fogg Point Freeport CBEP Priority Water Northeast Creek Bar Harbor DMR/NPS Threat Oakhurst Island Harpswell CBEP Priority Water Ogunquit River Estuary Ogunquit MHB Priority Water, DMR/NPS Threat Pemaquid River Bristol DMR/NPS Threat Salt Pond Blue Hill/Sedgwick DMR/NPS Threat, MERI Scarborough River Estuary Scarborough DMR/NPS Threat Spinney Creek Eliot MS4 Priority Water, Negative Water Quality Indicators Spruce Creek Kittery MS4 Priority Water, Negative Water Quality Indicators Page 1 of 2 MDEP NPS Priority Watersheds List – MARINE WATERS February 2019 Marine Water Area/Town Priority List Reasoning Spurwink River Scarborough MHB Priority Water, DMR/NPS Threat St. -

Phippsburg/FOMB Appeal of August Dredging 5-16-11
STATE OF MAINE BOARD OF ENVIRONMENTAL PROTECTION U.S. ARMY CORPS OF ENGINEERS ) NATURAL RESOURCES PROTECTION ACT Bath and Phippsburg, Sagadahoc County ) COASTAL WETLAND ALTERATION MAINTENANCE DREDGING ) WATER QUALITY CERTIFICATION L-16281-4E-E-N ) APPEAL OF THE DECISION OF THE COMMISSIONER APPROVING THE U.S. ARMY CORPS OF ENGINEERS KENNEBEC RIVER DREDGING PROJCT NOW COME the Town of Phippsburg, the Phippsburg Shellfish Conservation Commission, the Phippsburg Land Trust, the Kennebec Estuary Land Trust, the Friends of Merrymeeting Bay, Bob Cummings, Lawrence Pye, Dean Doyle, Dot Kelly, Captain Ethan DeBery, and Laura Sewall (together as “Appellants”) to appeal the decision of the Commissioner of the Maine Department of Environmental Protection (“Department” or “DEP”) on April 14, 2011 (“Order”), granting to the U.S. Army Corps of Engineers (“Corps”) a water quality certification pursuant to section 401 of the federal Clean Water Act (“401 Certification”) and a permit under the Natural Resources Protection Act (“NRPA”). The Order authorizes the Corps to conduct out-of-season maintenance and advanced maintenance dredging in August of two locations in the Kennebec River in Bath and Phippsburg, Maine, and to dump approximately 70,000 cubic yards of dredge spoils at two locations in the Kennebec River and near-shore environments in Phippsburg. For the reasons below, Appellants request that the Board of Environmental Protection (“Board”) modify the Order to allow only the minimum out-of-season August dredging necessary, if any, to allow the U.S.S. Spruance to safely transit the Kennebec River in September, and to require that disposal of dredge spoils, if any, occur at upland and/or offshore locations where it will not cause unreasonable impacts to the environment or to Appellants. -

Seawall and Popham Beach Dynamics, Phippsburg
Seawall and Popham Beach Dynamics Maine Geological Survey Maine Geologic Facts and Localities November, 2008 Seawall and Popham Beach Dynamics Phippsburg, Maine 43° 43‘ 56.57“ N, 69° 48‘ 37.22“ W Text by Stephen M. Dickson Maine Geological Survey, Department of Agriculture, Conservation & Forestry 1 Seawall and Popham Beach Dynamics Maine Geological Survey Introduction Beach dynamics and sand movement along the shoreline at Popham Beach State Park and Seawall Beach is a continuing saga of extreme shoreline change and dune erosion. Here we look at Seawall Beach, the Morse River, and the large sand spit (bar) connected to Seawall Beach that extends seaward of Popham Beach. In fall 2008 an enormous beach spit was connected to Seawall Beach reflecting several years of growth and easterly extension. It currently blocks the Morse River from flowing directly south to the sea and has led to severe dune erosion and loss of mature pitch pine trees in the back dune maritime forest at Popham Beach State Park. For more on the historical location of the Morse River and erosion at the park see the previous MGS web page, Tombolo Breach at Popham Beach State Park, Phippsburg, Maine. Maine Geological Survey, Department of Agriculture, Conservation & Forestry 2 Seawall and Popham Beach Dynamics Maine Geological Survey Hiking to Seawall Beach to Visit the Morse River Access to the mouth of the Morse River can be from the east via Popham Beach State Park or from the west via Seawall Beach. In order to see the new beach spit first-hand or to walk out on it when the tide is low, a hike from Route 216 will lead to the beach and the spit (Figure 1). -

Maine Healthy Beaches Program
Maine Healthyyg Beaches Program: The State of Maine’s Beaches in 2009 MiMaine Beach es C on ference July 10, 2009 Keri Lindberg Fundinggp provided by: US EPA Program Coordinator, UMaine Cooperative Extension/ Maine Sea Grant Sarah Mosley, Mark Margerum, Program Manager, UMaine Cooperative Extension ME Department of Environmental Protection Long Sands Beach, York, ME Geometric Mean 40 35 30 ls mm 25 20 mpn/100 15 10 5 0 2003 2004 2005 2006 2007 2008 YK-6 YK-13 YK-8 YK-16 YK-10 YK-18 YK-11 EPA Safety Level Long Sands Beach, York, ME Potential Bacteria Sources: 9Stormwater Runoff 9Residential/Commercial Development 9Boats 9Bathers 9Wildlife 9Pet Waste Cape Neddick Beach, York, ME Geometric Mean 40 35 30 25 20 pn/100 mls 15 m 10 5 0 2003 2004 2005 2006 2007 2008 YK-2 EPA Safety Level Cape Neddick Beach, York, ME Potential Bacteria Sources: Aliht&NtStAccomplishments & Next Steps: 9Cape Neddick River Outlet 9Town Hired a Shoreland 9Malfunctioning Septic Systems Resource Officer 9Stormwater Runoff 92007-2009 Cape Neddick River Special Study 9Residential/Commercial Development 9In 2008, Selectmen unanimously supported Plan of Work for 2009 9Boats 9Bathers 9Wildlife 9PtWPet Was te Ogunquit Beaches, Ogunquit, ME Geometric Mean 40 35 30 ls 25 20 15 mpn/100 m 10 5 0 2003 2004 2005 2006 2007 2008 OG-1 OG-4 OG-2 OG-5 OG-3 EPA Safety Level 2005 – 2009 Ogunquit River Special Study Potential Bacteria Sources: 9Ogunquit River Outlet 9Malfunctioning Septic Systems 9Stormwater Runoff 9Residential/Commercial Development 9Sanitary Sewer Outfall 9Bathers 9Wildlife 9Pet Waste 2005 – 2009 Ogunquit River Special Study 9Maine Geological Survey Acoustic Doppler Profiling Study & Data Analysis 94 separate current zones and 1 sub-zone 9Oggqunquit River = p rimar y source of contamination 9Wave ht >4 ft. -

IMPORTANT BIRD AREAS of MAINE an Analysis Of
IMPORTANT BIRD AREAS OF MAINE An Analysis of Avian Diversity and Abundance Compiled by: Susan Gallo, Thomas P. Hodgman, and Judy Camuso A Project Supported by the Maine Outdoor Heritage Fund IMPORTANT BIRD AREAS OF MAINE An Analysis of Avian Diversity and Abundance February 7, 2008 Compiled by: Susan Gallo, Maine Audubon, 20 Gilsland Farm Rd., Falmouth, ME 04105 Thomas P. Hodgman, Maine Department of Inland Fisheries and Wildlife, 650 State St., Bangor, ME 04401 Judy Camuso, Maine Audubon, 20 Gilsland Farm Rd., Falmouth, ME 04105 (Present Address: Maine Department of Inland Fisheries and Wildlife, 358 Shaker Road, Gray, ME 04039) Recommended citation: Gallo, S., T. P. Hodgman, and J. Camuso, Compilers. 2008. Important Bird Areas Of Maine: an analysis of avian diversity and abundance. Maine Audubon, Falmouth, Maine. 94pp. Cover Photo: Scarborough Marsh at sunrise, by W. G. Shriver ii Table of Contents History ..........................................................................................................................................1 What is an Important Bird Area?.......................................................................................1 Qualifying Criteria...................................................................................................................1 Data Use and Applicability Disclaimer .............................................................................2 Acknowledgements...................................................................................................................3 -

3. the Presence of Freshwater Cord Grass ~S Artina ~Sctinata!
Summary of Significant Features A, Geological 1. Fringing pocket beach with two central beach strea~ outlets non-tidal!. 2. Low sand budget with dramatic summer buildup resulting in a wide berm. 3. Stable shoreline position. 4. The southwestern end is the long-term downdrift end. This is indicated by the width of the back dune, but the beach has finer sand toward the north- east, suggesting short-term downdrift toward the northeast. 5. Good illustration of seasonal accretionary profile. 6. Height of the frontal dune ridge is a function of width of the berm more than a function of the direction it faces. 7. There are no parabolic dunes, but there are good dry dune flats with asso- ciated dune plant species in the back dune. B, Botanical I, The northern coastal range limit of Wormwood Artemisia caudata!. 2. Good Beach Heather Hudsonia tomentosa! patches and associated plants, 3. The presence of Freshwater Cord Grass ~Sartina ~sctinata!. 4. No pitch pines or semi-open community. Not a positive feature.! 5. Good vegetation cover, no foot traffic damage, well managed. C. Size Crescent Beach State Park covers an area of 31 hectares and has a length of 1524 m. Bailey Beach Phi sbur , Sagadahoc Count Description of Geological Features Bailey Beach Figure 27! is a small fringing pocket beach .4 hectares! with a relatively wide back dune area for such a short beach. A large volume of sand has been blown onshore to cover low-lying bedrock upland. Exposure of a coarse cobble/ boulder lag surface at the western end of the beach and the rapid grading to coarse sand beneath the lower beachface suggests that the shoreline has probably never been much further back than today, The sand appears to be locally derived and is not spillover from the Popham-Seawall system. -

Town of Ogunquit Comprehensive Plan Table of Contents
Town of Ogunquit Comprehensive Plan Table of Contents 1. Introduction of the Town 2. Population Inventory Goals Policies & Strategies 3. Local Economy Inventory Goals Policies & Strategies 4. Housing Inventory Goals Policies & Strategies 5. The Arts and Culture Goals Policies & Strategies 6. Transportation Goals Policies & Strategies 7. Conservation Goals Policies & Strategies 8. Sea Level Rise/Climate Change Goals Policies & Strategies 9. Tourism/Green Tourism Goals Policies & Strategies 10. Aging in Place Goals Policies & Strategies 11. Historic Preservation Goals Policies & Strategies 12. Land Use/Future Land Use Goals Policies & Strategies Dear Ogunquit Residents Welcome to the 2018 Comprehensive Plan Update! What is a Comprehensive Plan Update? So glad you asked! It is the long-range plan that will guide Ogunquit’s future for the next ten years. It is a legal document. The Comprehensive Plan is not itself a regulation; it does not make actual code or zoning changes. Rather, it provides a roadmap for the Town’s future that will recommend and inform regulations and code changes going forward. Many changes for the Town cannot be made unless they have been included in the Comprehensive Plan. The Plan is made up of goals, which are broad, over-arching ideas; policies which are objectives for reaching the goals; and strategies which are specific actions recommended to address the policies. Each strategy is assigned to a responsible party and given a timeline. As this Committee has learned, long-range planning for an entire town is a multi-layered and open- ended process. We have been at this since April of 2016 when we all thought we were in for a one- year term.