The Evaluation of Pituitary Damage Associated with Cardiac Arrest: an Experimental Rodent Model Yu Okuma1, Tomoaki Aoki1, Santiago J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1-Anatomy of the Pituitary Gland
Color Code Important Anatomy of Pituitary Gland Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of the lecture, students should be able to: ✓ Describe the position of the pituitary gland. ✓ List the structures related to the pituitary gland. ✓ Differentiate between the lobes of the gland. ✓ Describe the blood supply of pituitary gland & the hypophyseal portal system. الغدة النخامية Pituitary Gland (also called Hypophysis Cerebri) o It is referred to as the master of endocrine glands. o It is a small oval structure 1 cm in diameter. o It doubles its size during pregnancy. lactation ,(الحمل) pregnancy ,(الحيض) A women experiences changes in her hormone levels during menstruation But only the pituitary gland will only increase in size during pregnancy .(سن اليأس) and menopause ,(الرضاعة) X-RAY SKULL: LATERAL VIEW SAGITTAL SECTION OF HEAD & NECK Extra Pituitary Gland Position o It lies in the middle cranial fossa. o It is well protected in sella turcica* (hypophyseal fossa) of body of sphenoid o It lies between optic chiasma (anteriorly) & mamillary bodies** (posteriorly). Clinical point: *سرج الحصان Anterior to the pituitary gland is the optic chiasm, so if there was a tumor in the pituitary gland or it was ** Part of hypothalamus enlarged this could press on the chiasm and disrupt the patients vision (loss of temporal field). Extra Pictures The purple part is the sphenoid bone Hypophyseal fossa Pituitary Gland The relations are important Important Relations • SUPERIOR: Diaphragma sellae: A fold of dura mater covers the pituitary gland & has an opening for passage of infundibulum (pituitary stalk) connecting the gland to hypothalamus. -

Glossary of Pediatric Endocrine Terms
Glossary of Pediatric Endocrine Terms Adrenal Glands: Triangle-shaped glands located on top of each kidney that are responsible for the regulation of stress response by producing hormones such as cortisol and adrenaline. Adrenal Crisis: A life-threatening condition that results when there is not enough cortisol (stress hormone) in the body. This may present with nausea, vomiting, abdominal pain, severely low blood pressure, and loss of consciousness. Adrenocorticotropin (ACTH): A hormone produced by the anterior pituitary gland that triggers the adrenal gland to produce cortisol, the stress hormone. Anterior Pituitary: The front of the pituitary gland that secretes growth hormone, gonadotropins, thyroid stimulating hormone, prolactin, adrenocorticotropin hormone. Antidiuretic Hormone: A hormone secreted by the posterior pituitary that controls how much water is excreted by the kidneys (water balance). Bone Age Study: An X-ray of the left hand and wrist to assess a child’s skeletal age. It is often used to evaluate disorders of puberty and growth. Congenital Adrenal Hyperplasia: A group of disorders which impair the adrenal steroid synthesis pathway. This causes the body makes too many androgens. Sometimes the body does not make enough cortisol and aldosterone. Constitutional Delay of Growth and Puberty: A normal variant of growth in which children grow at a slower rate and begin puberty later than their peers. They may appear short until they have catch up growth. They usually end up at a normal adult height. A diagnosis of constitutional delay of growth and puberty is often referred to as being a “late bloomer.” Cortisol: The “stress hormone”, which is produced by the adrenal glands. -

Hypothalamus and Pituitary Gland Development in the Common Snapping Turtle, Chelydra Serpentina, and Disruption with Atrazine Exposure
University of North Dakota UND Scholarly Commons Theses and Dissertations Theses, Dissertations, and Senior Projects January 2016 Hypothalamus And Pituitary Gland Development In The ommonC Snapping Turtle, Chelydra Serpentina, And Disruption With Atrazine Exposure Kathryn Lee Gruchalla Russart Follow this and additional works at: https://commons.und.edu/theses Recommended Citation Russart, Kathryn Lee Gruchalla, "Hypothalamus And Pituitary Gland Development In The ommonC Snapping Turtle, Chelydra Serpentina, And Disruption With Atrazine Exposure" (2016). Theses and Dissertations. 2069. https://commons.und.edu/theses/2069 This Dissertation is brought to you for free and open access by the Theses, Dissertations, and Senior Projects at UND Scholarly Commons. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of UND Scholarly Commons. For more information, please contact [email protected]. HYPOTHALAMUS AND PITUITARY GLAND DEVELOPMENT IN THE COMMON SNAPPING TURTLE, CHELYDRA SERPENTINA, AND DISRUPTION WITH ATRAZINE EXPOSURE by Kathryn Lee Gruchalla Russart Bachelor of Science, Minnesota State University, Mankato, 2006 A Dissertation Submitted to the Graduate Faculty of the University of North Dakota in partial fulfillment of the requirements for the degree of Doctor of Philosophy Grand Forks, North Dakota August 2016 Copyright 2016 Kathryn Russart ii iii PERMISSION Title Hypothalamus and Pituitary Gland Development in the Common Snapping Turtle, Chelydra serpentina, and Disruption with Atrazine Exposure Department Biology Degree Doctor of Philosophy In presenting this dissertation in partial fulfillment of the requirements for a graduate degree from the University of North Dakota, I agree that the library of this University shall make it freely available for inspection. -

Pituitary Disease Handbook for Patients Disclaimer This Is General Information Developed by the Ottawa Hospital
The Ottawa Hospital Divisions of Endocrinology and Metabolism and Neurosurgery Pituitary Disease Handbook for Patients Disclaimer This is general information developed by The Ottawa Hospital. It is not intended to replace the advice of a qualified health-care provider. Please consult your health-care provider who will be able to determine the appropriateness of the information for your specific situation. Prepared by Monika Pantalone, NP Advanced Practice Nurse for Neurosurgery With guidance from Dr. Charles Agbi, Dr. Erin Keely, Dr. Janine Malcolm and The Ottawa Hospital pituitary patient advisors P1166 (10/2014) Printed at The Ottawa Hospital Outline This handbook is designed to help people who have pituitary tumours better understand their disease. It contains information to help people with pituitary tumours discuss their care with health care providers. This booklet provides: 1) An overview of what pituitary tumours are and how they are grouped 2) An explanation of how pituitary tumours are investigated 3) A description of available treatments for pituitary tumours What is the Pituitary Gland? The pituitary gland is a pea size organ located just behind the bridge of the nose at the base of the brain, in a bony pouch called the “sella turcica.” It sits just below the nerves to the eyes (the optic chiasm). The pituitary gland is divided into two main portions: the larger anterior pituitary (at the front) and the smaller posterior pituitary (at the back). Each of these portions has different functions, producing different types of hormones. Optic Pituitary Hypothalamus tumor chiasm Pituitary stalk Anterior Sphenoid pituitary gland sinus Posterior pituitary gland Sella Picture provided by turcica pituitary.ucla.edu 1 The pituitary gland is known as the “master gland” because it helps to control the secretion of various hormones from a number of other glands including the thyroid gland, adrenal glands, testes and ovaries. -

Pituitary Tumor-Transforming Gene in Endocrine and Other Neoplasms: a Review and Update
Endocrine-Related Cancer (2008) 15 721–743 REVIEW Pituitary tumor-transforming gene in endocrine and other neoplasms: a review and update Fateme Salehi1, Kalman Kovacs1, Bernd W Scheithauer 3, Ricardo V Lloyd 3 and Michael Cusimano2 Departments of 1Laboratory Medicine and 2Neurosurgery, St Michael’s Hospital, 30 Bond Street, Toronto, Ontario, Canada M5B 1W8 3Department of Laboratory Medicine and Pathology, Mayo Clinic, 200 First Street, SW, Rochester, Minnesota 55905, USA (Correspondence should be addressed to F Salehi; Email: [email protected]) Abstract Pituitary tumor-transforming gene (PTTG) was only recently discovered. Its overexpression occurs in a wide variety of endocrine and non-endocrine tumors, including ones of pituitary, thyroid, ovary, breast, prostate, lung, esophagus, colon, and the central nervous system. It affects tumor invasiveness and recurrence in several systems, functions as a securin during cell cycle progression, and inhibits premature sister chromatid separation. PTTG is involved in multiple cellular pathways, including proliferation, DNA repair, transformation, angiogenesis induction, invasion, and the induction of genetic instability. In thyroid carcinomas, PTTG expression is a marker of invasiveness. PTTG is overexpressed in most pituitary adenomas, where it appears to correlate with recurrence and angiogenesis. Increasing evidence also points to the role of PTTG in endocrine organ development. For example, PTTG knockout mice show defective pancreatic b-cell proliferation. Herein, we review the current knowledge regarding PTTG-mediated pathways based on evidence from in vivo and in vitro studies as well as knockout mice models. We also summarize the issue of PTTG expression and its correlation with clinicopathologic parameters in patients with neoplasms, particularly of endocrine organs. -

Chapter 5: Responding to the World
OXFORD BIG IDEAS SCIENCE 9: AUSTRALIAN CURRICULUM 5 RESPONDING TO THE WORLD 134 STRUCTURE AND FUNCTION 135 Each unit in this chapter opens with 5.3 What is a nervous response? an engaging image, supported by text <<BIG IDEAS>> Structure and function 1 Student responses will vary; however, the and questions. These can stimulate human body’s re exes and responses would Human presence causes major changes to the Earth, but how discussion, helping teachers to have helped them survive, regardless of the does our environment infl uence us? How does your body interact determine what students know and to Responding with the world around it? This chapter will open your eyes to the situation. give them an idea of the chapter theme. fi ne balance that must be maintained by your body to survive. 2 Student responses will vary. Examples of re exes 5 It is about how your body senses changes and threats and how it This encourages a self-directed inquiry include kicking out when a doctor taps your to the world responds to ensure you remain healthy. approach to learning. knee with a small hammer, pulling your hand away when you touch a hot kettle, and blinking What is a ➔ Fig 5.3 Our refl exes give us the ability to act when something suddenly comes very close to 5.3 and respond quickly. Curriculum guidance 5.2 hormonal What is a nervous response? your face. response? 3 If re exes didn’t occur, the body may be injured Previous Year 9 One ability of most superheroes is their amazingly 1 Think of a story you have heard or face some other type of danger. -

Pituitary Metastasis Unveiling a Lung Adenocarcinoma
ID: 20-0211 -20-0211 A M Lopes and others Pituitary metastasis and lung ID: 20-0211; February 2021 adenocarcinoma DOI: 10.1530/EDM-20-0211 Pituitary metastasis unveiling a lung adenocarcinoma Ana M Lopes 1, Josué Pereira2, Isabel Ribeiro3, Ana Martins da Silva4,5, Henrique Queiroga6,7 and Cláudia Amaral1 1Endocrinology Department, Centro Hospitalar e Universitário do Porto, EPE, Porto, Portugal, 2Neurosurgery Correspondence Department, Centro Hospitalar de São João, EPE, Porto, Portugal, 3Neurosurgery Department, 4Neurology Department, should be addressed Centro Hospitalar e Universitário do Porto, EPE, Porto, Portugal, 5Unidade Multidisciplinar de Investigação Biomédica, to A M Lopes Instituto de Ciências Biomédicas Abel Salazar, Universidade do Porto, Portugal, 6Pulmonology Department, Email Centro Hospitalar de São João, EPE, Porto, Portugal, and 7Faculty of Medicine, Porto University, Porto, Portugal [email protected] Summary Pituitary metastasis (PM) can be the initial presentation of an otherwise unknown malignancy. As PM has no clinical or radiological pathognomonic features, diagnosis is challenging. The authors describe the case of a symptomatic PM that revealed a primary lung adenocarcinoma. A 62-year-old woman with multiple sclerosis and no history of malignancy, incidentallypresentedwithadiffuselyenlargedandhomogeneouslyenhancingpituitaryglandassociatedwithstalk enlargement. Clinical and biochemical evaluation revealed anterior hypopituitarism and diabetes insipidus. Hypophysitis was considered the most likely diagnosis. However, rapid visual deterioration and pituitary growth raised the suspicion of metastatic involvement. A search for systemic malignancy was performed, and CT revealed a lung mass, which proved to be a lung adenocarcinoma. Accordingly, the patient was started on immunotherapy. Resection of the pituitary lesion was performed, and histopathology analysis revealed metastatic lung adenocarcinoma. Following surgery, the patient underwent radiotherapy. -
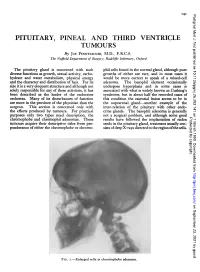
PITUITARY, PINEAL and THIRD VENTRICLE TUMOURS by JOE PENNYIIACKER, M.D., F.R.C.S
'4I Postgrad Med J: first published as 10.1136/pgmj.26.293.141 on 1 March 1950. Downloaded from PITUITARY, PINEAL AND THIRD VENTRICLE TUMOURS By JOE PENNYIIACKER, M.D., F.R.C.S. The Nuffield Department of Surgery, Radcliffe Infirmary, Oxford The pituitary gland is concerned with such phil cells found in the normal gland, although pure diverse functions as growth, sexual activity, carbo- growths of either are rare, and in most cases it hydrate and water metabolism, physical energy would be more correct to speak of a mixed-cell and the character and distribution of hair. For its adenoma. The basophil element occasionally size it is a very eloquent structure and although not undergoes hyperplasia and in some cases is solely responsible for any of these activities, it has associated with what is widely known as Cushing's been described as the leader of the endocrine syndrome, but in about half the recorded cases of orchestra. Many of its disturbances of function this condition the essential lesion seems to be in are more in the province of the physician than the the suprarenal gland-another example of the surgeon. This section is concerned only with inter-relation of the pituitary with other endo- the effects produced by tumours. For practical crine glands. The basophil adenoma is generally purposes only two types need description, the not a surgical problem, and although some good Protected by copyright. chromophobe and chromophil adenomas. These results have followed the implantation of radon tumours acquire their descriptive titles from pre- seeds in the pituitary gland, treatment usually con- ponderance of either the chromophobe or chromo- sists ofdeep X-rays directed to the region ofthe sella. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -

The Digestive and Endocrine Systems Examination
The Digestive and Lesson 3 Endocrine Systems Lesson 3 ASSIGNMENT 6 Read in your textbook, Clinical Anatomy and Physiology for Veterinary Technicians, pages 358–377, 436, and 474–475. Then read Assignment 6 in this study guide. Introduction The endocrine system involves the secretion of chemicals called hormones by glands within the body. These chemicals control bodily functions, often at locations very distant from the gland that secreted the chemical. The opposite of endocrine is exocrine, which involves the secretion of sub- stances into spaces outside the body. The glands in the skin and gastrointestinal tract are examples of exocrine organs. (The lumen of the intestine is a space that technically isn’t “within” the body, because it’s continuous with the outside environment via the mouth and anus.) The pancreas is unique in being both an endocrine gland and an exocrine gland. The endocrine function is the secretion of substances such as insulin, which metabolizes sugar. The exocrine function involves the secretion of digestive enzymes into the duodenum. Many endocrine organs exist throughout the body (see Table 15-2 on page 360 of your textbook). While they’re all classified as endocrine glands, their anatomy and functions aren’t necessarily similar or even remotely related. Therefore, there isn’t really a grand organizational scheme to this sys- tem as there is with some of the other body systems. The glands are considered together only because their means of secretion is similar. All endocrine glands secrete hormones in the form of proteins that travel via the blood to the target organ for that particular hormone. -

The Respiratory System Pharyngeal Tonsil the Respiratory System Is Associated with Breathing
EMT-I_Ch05_114-239 9/25/04 1:34 PM Page 201 Chapter 5 The Human Body 201 The Respiratory System Pharyngeal tonsil The respiratory system is associated with breathing, Palatoglossal gas exchange, and the entrance of air into the body. The arch organs and structures in the respiratory system are Uvula divided into the upper and lower airways. The upper airway includes the mouth, nasal cavity, and oral cav- Palatine tonsil ity, and the lower airway includes the larynx, trachea, Palatopharyngeal bronchi, bronchioles, and alveoli. arch Lingual tonsil The Upper Airway Figure 5-111 The tonsils. A human can breathe air into the body through either the nose, also called the nasal cavity or nasopharynx, or through the mouth, also called the oral cavity or quite large in the infant and decreases in size with oropharynx. The nasopharynx and oropharynx connect age. Often, there is no discernible thymic tissue in posteriorly to form a cavity called the pharynx older adults. The thymus produces lymphocytes, which Figure 5-112 ᮢ . move to other lymph tissues to help fight infection. The nasopharynx extends from the internal nares to The thymus plays a major role in immunity, especially the uvula (a small fleshy mass that hangs from the soft in early life. palate). The oropharynx extends from the uvula to the A Artery Vein Alveolus Nasal cavity Bronchiole Mouth (oral cavity) Pharynx Larynx Capillary network Trachea Left bronchus Right bronchus B Alveoli Terminal bronchiole Capillaries Figure 5-112 The respiratory system. A. The upper and lower airway divisions of the respiratory system. The insert shows a higher magnification of the alveoli, where oxygen and carbon dioxide exchange occurs. -

The Endocrine System Dr
The Endocrine System Dr. Ali Ebneshahidi Copyright © 2006 Pearson Education, Inc., publishing as Benjamin Cummings Endocrine System . The endocrine system interacts with the nervous system to coordinate and integrate body activities by means of hormones . Endocrine tissues and organs secrete hormone into body fluids (mainly blood and lymph) directly using diffusion. Exocrine tissues, such as salivary glands, and sebaceous glands, secrete chemical substances through ducts into an open space. Copyright © 2006 Pearson Education, Inc., publishing as Benjamin Cummings Five major functions of hormones . a) Regulate metabolic processes (e.g. thyroid hormones). b) Control the rate of chemical reactions (e.g. growth hormone). c) Aid in the transport of substances across the cell membrane of target cells (e.g. insulin and glucagon). d) Regulate water and electrolyte balances (e.g. antidiurectic hormone, calcitonin, and aldosterone). e) Play a vital role in reproduction, growth and development (e.g. estrogens , progesterone, and testosterone). Copyright © 2006 Pearson Education, Inc., publishing as Benjamin Cummings Major Endocrine Organs Copyright © 2006 Pearson Education, Inc., publishing as Benjamin Cummings Chemistry of Hormones . Hormones are organic compounds secreted by endocrine glands, that have a potent effect in target cells Two types of hormones: . a) Protein hormones: made of amino acids joined by peptide bonds. fat – insoluble; as a result cannot diffuse across the membrane of target cells . most hormones belong to this group except hormones secreted by the gonads (testis and ovary) and the adrenal cortex. b) Steroid hormones: made of fatty acids using cholesterol as a functional group. Fat-soluble; as a result can diffuse into target cells .