A New Trick for an Old Lipid Cholesterol Can Regulate the Hedgehog Signalling Pathway by Directly Binding to a Receptor on the Cell Surface
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coincident Two Mutations and One Single Nucleotide Polymorphism of the PTCH1 Gene in a Family with Naevoid Basal Cell Carcinoma Syndrome
Letters to the Editor 635 Coincident Two Mutations and One Single Nucleotide Polymorphism of the PTCH1 Gene in a Family with Naevoid Basal Cell Carcinoma Syndrome Shoko Abe1, Kenji Kabashima1*, Jun-ichi Sakabe1, Takatoshi Shimauchi1, Zhang Yan2, Tetsuji Okamoto2 and Yoshiki Tokura1 1Department of Dermatology, University of Occupational and Environmental Health, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu 807-8555, and 2Department of Molecular Oral Medicine and Maxillofacial Surgery 1, Division of Frontier Medical Science, Graduate School of Biomedical Sciences, Hiroshima University, Hiroshima, Japan. E-mail: [email protected] Accepted May 7, 2008. Sir, at nucleotide position 667 within exon 3 and an intervening se- Naevoid basal cell carcinoma syndrome (NBCCS, OMIM quence (IVS)16 -3T > C, and a SNP; IVS10 -8T > C, were detected in all three cases. A deletion of AGAC causes a frameshift and a #109400), also called Gorlin’s syndrome, is an autosomal subsequent stop codon in exon 3, which prematurely truncates dominant disease that affects about 1 in 60,000 indivi- the protein (Fig. 1a). In addition, the IVS16 -3T > C could lead duals (1, 2). NBCCS is associated with various skeletal to an aberrant splicing and truncation of PTCH1 (10–12). and neurocutaneous abnormalities. Major manifestations To detect the expression level of PTCH1 protein in the skin, are multiple basal cell carcinomas (BCCs), odontogenic an immunohistochemical study was performed using goat poly- clonal anti-PTCH1 antibody (G-19; Santa Cruz Biotechnology, keratocysts, palmoplantar dyskeratotic pits and intra- Santa Cruz, CA, USA). Enzyme reactions were developed with cranial calcification (3). In addition, rib and vertebral conventional substrates for diamino-benzidine (Sigma, St Louis, malformations, epidermal cysts, macrocephaly, facial MO, USA) (13). -

Review of the Molecular Genetics of Basal Cell Carcinoma; Inherited Susceptibility, Somatic Mutations, and Targeted Therapeutics
cancers Review Review of the Molecular Genetics of Basal Cell Carcinoma; Inherited Susceptibility, Somatic Mutations, and Targeted Therapeutics James M. Kilgour , Justin L. Jia and Kavita Y. Sarin * Department of Dermatology, Stanford University School of Medcine, Stanford, CA 94305, USA; [email protected] (J.M.K.); [email protected] (J.L.J.) * Correspondence: [email protected] Simple Summary: Basal cell carcinoma is the most common human cancer worldwide. The molec- ular basis of BCC involves an interplay of inherited genetic susceptibility and somatic mutations, commonly induced by exposure to UV radiation. In this review, we outline the currently known germline and somatic mutations implicated in the pathogenesis of BCC with particular attention paid toward affected molecular pathways. We also discuss polymorphisms and associated phenotypic traits in addition to active areas of BCC research. We finally provide a brief overview of existing non-surgical treatments and emerging targeted therapeutics for BCC such as Hedgehog pathway inhibitors, immune modulators, and histone deacetylase inhibitors. Abstract: Basal cell carcinoma (BCC) is a significant public health concern, with more than 3 million cases occurring each year in the United States, and with an increasing incidence. The molecular basis of BCC is complex, involving an interplay of inherited genetic susceptibility, including single Citation: Kilgour, J.M.; Jia, J.L.; Sarin, nucleotide polymorphisms and genetic syndromes, and sporadic somatic mutations, often induced K.Y. Review of the Molecular Genetics of Basal Cell Carcinoma; by carcinogenic exposure to UV radiation. This review outlines the currently known germline and Inherited Susceptibility, Somatic somatic mutations implicated in the pathogenesis of BCC, including the key molecular pathways Mutations, and Targeted affected by these mutations, which drive oncogenesis. -

The Cell Adhesion Molecule CHL1 Interacts with Patched-1 to Regulate
© 2017. Published by The Company of Biologists Ltd | Journal of Cell Science (2017) 130, 2606-2619 doi:10.1242/jcs.194563 RESEARCH ARTICLE The cell adhesion molecule CHL1 interacts with patched-1 to regulate apoptosis during postnatal cerebellar development Jelena Katic1, Gabriele Loers1, Jelena Tosic1, Melitta Schachner2,3,4,* and Ralf Kleene1 ABSTRACT differentiated neural cells (Holm et al., 1996; Chen et al., 1999; The immunoglobulin superfamily adhesion molecule close homolog of Hillenbrand et al., 1999; Buhusi et al., 2003; Jakovcevski et al., L1 (CHL1) plays important roles during nervous system development. 2007, 2009; Katic et al., 2014). Moreover, CHL1 regulates Here, we identified the hedgehog receptor patched-1 (PTCH1) as a neuritogenesis through different mechanisms (Chen et al., 1999; novel CHL1-binding protein and showed that CHL1 interacts with Hillenbrand et al., 1999; Jakovcevski et al., 2007, 2009; Katic et al., the first extracellular loop of PTCH1 via its extracellular domain. 2014). In search for novel binding partners for CHL1, we found that trans Colocalization and co-immunoprecipitation of CHL1 with PTCH1 PTCH1 and CHL1 associate in a heterophilic -interaction. The suggest an association of CHL1 with this major component of the 12-pass transmembrane protein PTCH1 is a cognate receptor for the hedgehog signaling pathway. The trans-interaction of CHL1 with PTCH1 three mammalian hedgehog family members sonic hedgehog promotes neuronal survival in cultures of dissociated cerebellar granule (SHH), desert hedgehog (DHH) and indian hedgehog (IHH), cells and of organotypic cerebellar slices. An inhibitor of the PTCH1- which act as morphogens and function as signaling molecules by regulated hedgehog signal transducer, smoothened (SMO), and binding to PTCH1 (for reviews and references therein, see Jenkins, inhibitors of RhoA and Rho-associated kinase (ROCK) 1 and 2 2009; Robbins et al., 2012; Briscoe and Thérond, 2013). -

Underestimated PTCH1 Mutation Rate in Sporadic Keratocystic Odontogenic Tumors Q
Oral Oncology 51 (2015) 40–45 Contents lists available at ScienceDirect Oral Oncology journal homepage: www.elsevier.com/locate/oraloncology Underestimated PTCH1 mutation rate in sporadic keratocystic odontogenic tumors q Jiafei Qu a, Feiyan Yu a, Yingying Hong a, Yanyan Guo a, Lisha Sun b, Xuefen Li b, Jianyun Zhang a, ⇑ ⇑ Heyu Zhang b, Ruirui Shi b, Feng Chen b, , Tiejun Li a, a Department of Oral Pathology, Peking University School and Hospital of Stomatology, Beijing, China b Central Laboratory, Peking University School and Hospital of Stomatology, Beijing, China article info summary Article history: Objectives: Keratocystic odontogenic tumors (KCOTs) are benign cystic lesions of the jaws that occur spo- Received 11 July 2014 radically in isolation or in association with nevoid basal cell carcinoma syndrome (NBCCS). The protein Received in revised form 29 August 2014 patched homolog 1 gene (PTCH1) is associated with NBCCS development and tumor genesis associated Accepted 26 September 2014 with this syndrome. However, previous studies have revealed that more than 85% of syndromic KCOTs Available online 18 November 2014 and less than 30% of sporadic KCOTs harbor PTCH1 mutations. The significantly lower PTCH1 mutation rates observed in sporadic KCOTs suggest that they serve a minor role in pathogenesis. We aimed to Keywords: discern the importance of PTCH1 mutations in sporadic KCOTs. PTCH1 Materials and methods: PTCH1 mutational analysis was performed with 19 new sporadic KCOT cases by Mutation Keratocystic odontogenic tumors direct sequencing of epithelial lining samples separated from fibrous capsules. Using this approach, we further reexamined 9 sporadic KCOTs that were previously reported to lack PTCH1 mutations by our group. -

Nevoid Basal Cell Carcinoma Syndrome and Non-Hodgkin's
Nevoid Basal Cell Carcinoma Syndrome and Non-Hodgkin’s Lymphoma CPT Beth A. Schulz-Butulis, MC, USAR, Fort Sam Houston, Texas MAJ Robert Gilson, MC, USAF, Fort Sam Houston, Texas MAJ Mary Farley, MC, USAR, Fort Sam Houston, Texas COL James H. Keeling, MC, USA, Fort Sam Houston, Texas Nevoid basal cell carcinoma syndrome (NBCCS) is a hereditary disorder with a predilection for nu- merous basal cell carcinomas in addition to odon- togenic keratocysts, palmoplantar pitting, and skeletal malformations. NBCCS has been associ- ated with a number of benign and malignant neo- plasms. We report the first case of NBCCS in as- sociation with non-Hodgkin’s lymphoma. evoid basal cell carcinoma syndrome (NBCCS), also known as basal cell nevus syn- drome, Gorlin syndrome, Gorlin-Goltz syn- N 1,2 drome, or fifth phacomatosis, is an autosomal dom- inant disease linked to chromosome 9q22.3-q31.3-5 It is a progressive degenerative multisystem disease FIGURE 1. The back of a patient shows multiple basal characterized by a predisposition to develop basal cell cell carcinomas. (Photograph courtesy of MAJ Thomas carcinomas (BCCs) of the skin, palmoplantar pitting, Hirota, MC, USA.) odontogenic keratocysts, bifid ribs, kyphoscoliosis, short fourth metacarpals, ocular abnormalities, calci- the first known report of non-Hodgkin’s lymphoma fied dural folds, endocrine abnormalities, and various associated with NBCCS. internal neoplasms.1,2 Internal neoplasms that have been reported in asso- Case Report ciation with NBCCS include medulloblastoma,6 cran- A 54-year-old white man presented with numerous iopharyngioma,7 meningioma,2 astrocytoma,8 oligo- basal cell cancers. -

Characterization of Two Patched Receptors for the Vertebrate Hedgehog Protein Family
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 13630–13634, November 1998 Cell Biology Characterization of two patched receptors for the vertebrate hedgehog protein family DAVID CARPENTER*, DONNA M. STONE†,JENNIFER BRUSH‡,ANNE RYAN§,MARK ARMANINI†,GRETCHEN FRANTZ§, ARNON ROSENTHAL†, AND FREDERIC J. DE SAUVAGE*¶ Departments of *Molecular Oncology, ‡Molecular Biology, §Pathology, and †Neuroscience, Genentech Inc., 1 DNA Way, South San Francisco, CA 94080 Communicated by David V. Goeddel, Tularik, Inc., South San Francisco, CA, September 24, 1998 (received for review June 12, 1998) ABSTRACT The multitransmembrane protein Patched mammalian hedgehogs or whether ligand-specific components (PTCH) is the receptor for Sonic Hedgehog (Shh), a secreted exist. Interestingly, a second murine PTCH gene, PTCH2, was molecule implicated in the formation of embryonic structures isolated recently (25) but its function as a hedgehog receptor and in tumorigenesis. Current models suggest that binding of has not been established. To characterize PTCH2 and com- Shh to PTCH prevents the normal inhibition of the seven- pare it with PTCH with respect to the biological function of the transmembrane-protein Smoothened (SMO) by PTCH. Ac- various hedgehog family members, we isolated the human cording to this model, the inhibition of SMO signaling is PTCH2 gene. Binding analysis shows that both PTCH and relieved after mutational inactivation of PTCH in the basal PTCH2 bind to all three hedgehog ligands with similar affinity. cell nevus syndrome. Recently, PTCH2, a molecule with Furthermore PTCH2 interacts with SMO, suggesting that it sequence homology to PTCH, has been identified. To charac- can form a functional multicomponent hedgehog receptor terize both PTCH molecules with respect to the various complex similar to PTCH-SMO. -
Patched 1 and Patched 2 Redundancy Has a Key Role In
ORIGINAL ARTICLE Patched 1 and Patched 2 Redundancy Has a Key Role in Regulating Epidermal Differentiation Christelle Adolphe1,5, Erica Nieuwenhuis2,5, Rehan Villani1,5,ZhuJuanLi2,3, Pritinder Kaur4,Chi-chungHui2,3 and Brandon J. Wainwright1 The Patched 1 (Ptch1) receptor has a pivotal role in inhibiting the activity of the Hedgehog (Hh) pathway and is therefore critical in preventing the onset of many human developmental disorders and tumor formation. However, the functional role of the mammalian Ptch2 paralogue remains elusive, particularly the extent to which it contributes to regulating the spatial and temporal activity of Hh signaling. Here we demonstrate in three independent mouse models of epidermal development that in vivo ablation of both Ptch receptors results in a more severe phenotype than loss of Ptch1 alone. Our studies indicate that concomitant loss of Ptch1 and Ptch2 activity inhibits epidermal lineage specification and differentiation. These results reveal that repression of Hh signaling through a dynamic Ptch regulatory network is a crucial event in lineage fate determination in the skin. In general, our findings implicate Ptch receptor redundancy as a key issue in elucidating the cellular origin of Hh-induced tumors. Journal of Investigative Dermatology (2014) 134, 1981–1990; doi:10.1038/jid.2014.63; published online 27 February 2014 INTRODUCTION cells by binding to the Ptch1 receptor and stimulating The Hedgehog (Hh) signaling pathway has a central role in internalization and lysosomal degradation of the Hh–Ptch1 regulating appropriate embryonic development and maintain- complex (Mastronardi et al., 2000), thus relieving the repres- ing adult tissue homeostasis. Disruption to the spatiotemporal sive effect on Smo. -

Hedgehog Signaling Pathway
Available online a t www.scholarsresearchlibrary.com Scholars Research Library Annals of Biological Research, 2010, 1 (4) : 73-84 (http://scholarsresearchlibrary.com/archive.html) ISSN 0976-1233 CODEN (USA): ABRNBW Hedgehog signaling pathway Anil Reddy Marasani 1 * , Radhika Anakonti 1, Deepthi Rudrapati 2, Rosi reddy 2 3 Jonnavarapu , Basani Gavaskar 1Department of Pharmacology, St. Peter’s Institute Of Pharmaceutical Sciences, Hanamkonda, Warangal, A.P. 2Department of Pharmacology,Sri Venkateswara university, Tirupati, Chittoor, A.P. 3 Department of Pharmaceutics, Vagdevi college Of Pharmacy, Hanamkonda, Warangal, A.P. ______________________________________________________________________________ ABSTRACT The control and mediation of the cell cycle is influenced by cell signals. Different types of cell signaling molecules: Proteins (growth factors), peptide hormones, amino acids, steroids, retenoids, fatty acid derivatives, and small gases can all act as signaling molecules. The hedgehog signaling pathway is one of the key regulators of animal development conserved from flies (wing development in Drosophila) to humans (development of the brain, GI tract, fingers and toes in mammals). The pathway name is from its polypeptide ligand, an intercellular signaling molecule called Hedgehog (Hh) found in fruit flies of the genus Drosophila. Mutations or other sorts of regulatory errors in the hedgehog pathway are associated with a number of birth defects as well as some cancers. In this report, we will review the role of Hedgehog in cell signaling and impact of it in clinical medicine. Key words: Hedgehog, Metamorphosis, SMO, PTCH, SHH, Gorlin syndrome. ______________________________________________________________________________ INTRODUCTION Cell signaling is a complex system of communication, which controls and governs basic cellular activities and coordinates cell actions [1]. In biology point of view signal transduction refers to any process through which a cell converts one kind of signal or stimulus into another. -

Understanding the Hedgehog Signaling Pathway in Acute Myeloid Leukemia Stem Cells: a Necessary Step Toward a Cure
biology Review Understanding the Hedgehog Signaling Pathway in Acute Myeloid Leukemia Stem Cells: A Necessary Step toward a Cure Daniel Lainez-González 1 , Juana Serrano-López 1 and Juan Manuel Alonso-Domínguez 1,2,* 1 Experimental Hematology, Instituto de Investigación Sanitaria Fundación Jiménez Díaz, Universidad Autónoma de Madrid, Avenida Reyes Católicos 2, 28040 Madrid, Spain; [email protected] (D.L.-G.); [email protected] (J.S.-L.) 2 Hematology Department, Hospital Universitario Fundación Jiménez Díaz, Avenida Reyes Católicos 2, 28040 Madrid, Spain * Correspondence: [email protected]; Tel.: +34-918488100-2673 Simple Summary: The Hedgehog signaling pathway is related to the cell cycle. In particular, it is considered to play a fundamental role in the quiescence of leukemic stem cell (i.e., a temporary resting state without cell replication). Leukemic stem cells are the cells supposed to give rise to the relapses of the leukemia. Therefore, the Hedgehog pathway must be understood to improve the current treatments against acute myeloid leukemia and avoid the relapse of the disease. In this review, we gather the present knowledge about the physiological Hedgehog pathway function, the aberrant activation of Hedgehog in leukemia, and highlight the lack of evidence regarding some aspects of this important pathway. Finally, we summarize the acute myeloid leukemia treatments targeting this signaling pathway. Citation: Lainez-González, D.; Abstract: A better understanding of how signaling pathways govern cell fate is fundamental to Serrano-López, J.; advances in cancer development and treatment. The initialization of different tumors and their Alonso-Domínguez, J.M. maintenance are caused by the deregulation of different signaling pathways and cancer stem cell Understanding the Hedgehog maintenance. -
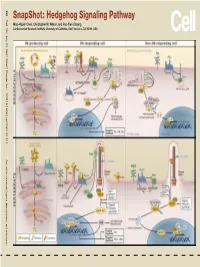
Snapshot: Hedgehog Signaling Pathway Miao-Hsueh Chen, Christopher W
SnapShot: Hedgehog Signaling Pathway Signaling SnapShot:Hedgehog Wilson, and Pao-Tien Chuang Miao-Hsueh Chen, Christopher W. Institute, University of California, Research San Francisco, CA 94158, USA Cardiovascular 386 Cell 130, July 27, 2007 ©2007 Elsevier Inc. DOI 10.1016/j.cell.2007.07.017 See online version for legend, abbreviations, and references. SnapShot: Hedgehog Signaling Pathway Miao-Hsueh Chen, Christopher W. Wilson, and Pao-Tien Chuang Cardiovascular Research Institute, University of California, San Francisco, CA 94158, USA The Hedgehog (Hh) signal transduction pathway controls numerous processes during fly and vertebrate embryonic development and adult homeostasis, including tissue/organ patterning, cellular proliferation and differentiation, pathfinding, left/right asymmetry, and stem cell maintenance. Hh signaling is dysregulated in several congenital defects and many types of tumors. Hh is able to signal at short-range but also may act as a long-range morphogen in multiple contexts (this aspect is controlled in part by lipid modification of the Hh molecule). Hh signaling controls the balance of the transcriptional activator and repressor forms of Ci/Gli, the ratios of which are essential for interpreting the level of Hh signal in a morphogenetic field and for generating diverse outputs. Hh-Producing Cell After translation, the Hh precursor enters the ER/Golgi secretory pathway. Autoproteolysis mediated by the C-terminal of Hh (Hh-C) releases the N-terminal signaling peptide (Hh-N), with cholesterol covalently linked to its C terminus. Addition of a palmitate moiety to the N terminus of the Hh signaling fragment is catalyzed by Skinny hedgehog (Ski/Skn). Lipid modifications are essential for the Hh ligand to induce high-level signaling activity and proper formation of a morphogen gradient. -

Smoothened Regulation in the Hedgehog Signaling Pathway
Smoothened regulation in the Hedgehog signaling pathway The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Nedelcu, Daniel. 2013. Smoothened regulation in the Hedgehog signaling pathway. Doctoral dissertation, Harvard University. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:11181143 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Smoothened regulation in the Hedgehog signaling pathway A dissertation presented by Daniel Nedelcu to The Division of Medical Sciences in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the subject of Cell Biology Harvard University Cambridge, Massachusetts August 2013 © 2013 Daniel Nedelcu All rights reserved. Thesis Advisor: Adrian Salic Daniel Nedelcu Abstract Hedgehog signaling is a pathway essential in embryonic development, adult stem cell maintenance, and is implicated in the formation and progression of cancer. Signaling in this pathway is triggered when the secreted protein Hedgehog binds to its membrane receptor, Patched. Patched normally inhibits the seven-spanner transmembrane protein Smoothened (Smo). Binding of Hedgehog inhibits Patched resulting in Smo derepression. Active Smo then triggers the activation of the cytoplasmic steps of the signaling pathway. The regulation of Smo is poorly understood mechanistically. Oxysterols bind Smo and potently activate vertebrate Hedgehog signaling. However, it is unknown if oxysterols are important for normal Hedgehog signaling, and whether antagonizing oxysterols can inhibit the Hedgehog pathway. -

Identification of Mutations in the Human PATCHED Gene In
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Identification of Mutations in the Human PATCHED Gene in Sporadic Basal Cell Carcinomas and in Patients with the Basal Cell Nevus Syndrome Michelle Aszterbaum, Alana Rothman, Ronald L. Johnson,* Monte Fisher, Jingwu Xie, Jeannette M. Bonifas, Xioli Zhang, Mathew P. Scott,* and Ervin H. Epstein Jr Department of Dermatology, University of California, San Francisco, California, U.S.A.; *Departments of Developmental Biology and Genetics, Howard Hughes Medical Institute, Stanford University School of Medicine, Stanford, California, U.S.A. Mutations in PATCHED (PTC), the human homolog of syndrome-associated basal cell carcinomas. This screen the Drosophila patched gene, have been identified in identified mutations located in eight exons in 13 of the most exons of the gene in patients with the basal cell basal cell nevus syndrome patients and in three of the nevus syndrome and in sporadic basal cell carcinomas. tumors. The most common mutations were frameshifts We have screened the 23 PTC exons for mutations using resulting in premature chain termination. These results single strand conformation polymorphism analysis of provide further evidence for the crucial role of PTC as DNA from 86 basal cell nevus syndrome probands, 26 a tumor suppressor in human keratinocytes. Keyword: sporadic basal cell carcinomas, and seven basal cell nevus Hedgehog. J Invest Dermatol 110:885–888, 1998 asal cell carcinomas (BCC) are the commonest human Marigo et al, 1996) or following mutational inactivation of Ptc. Smo cancer, with an estimated 750,000 cases per year in the signaling activates transcription of Hh targets including PTC, and there U.S.A.