Importance of Ice Algal Production for Top Predators: New Insights Using Sea-Ice Biomarkers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Management Plan for Antarctic Specially Protected Area No
Measure 2 (2005) Annex E Management Plan for Antarctic Specially Protected Area No. 120 POINTE-GÉOLOGIE ARCHIPELAGO, TERRE ADÉLIE Jean Rostand, Le Mauguen (former Alexis Carrel), Lamarck and Claude Bernard Islands, The Good Doctor’s Nunatak and breeding site of Emperor Penguins 1. Description of Values to be Protected In 1995, four islands, a nunatak and a breeding ground for emperor penguins were classified as an Antarctic Specially Protected Area (Measure 3 (1995), XIX ATCM, Seoul) because they were a representative example of terrestrial Antarctic ecosystems from a biological, geological and aesthetics perspective. A species of marine mammal, the Weddell seal (Leptonychotes weddelli) and various species of birds breed in the area: emperor penguin (Aptenodytes forsteri); Antarctic skua (Catharacta maccormicki); Adélie penguins (Pygoscelis adeliae); Wilson’s petrel (Oceanites oceanicus); giant petrel (Macronectes giganteus); snow petrel (Pagodrama nivea), cape petrel (Daption capense). Well-marked hills display asymmetrical transverse profiles with gently dipping northern slopes compared to the steeper southern ones. The terrain is affected by numerous cracks and fractures leading to very rough surfaces. The basement rocks consist mainly of sillimanite, cordierite and garnet-rich gneisses which are intruded by abundant dikes of pink anatexites. The lowest parts of the islands are covered by morainic boulders with a heterogenous granulometry (from a few cm to more than a m across). Long-term research and monitoring programs of birds and marine mammals have been going on for a long time already (since 1952 or 1964 according to the species). A database implemented in 1981 is directed by the Centre d'Etudes Biologiques de Chize (CEBC-CNRS). -

Part 4 Appendices
Part 4 Appendices HEARD ISLAND AND MCDONALD ISLANDS MARINE RESERVE 139 Appendix 1. Proclamation of Heard Island and McDonald Islands Marine Reserve 140 MANAGEMENT PLAN HEARD ISLAND AND MCDONALD ISLANDS MARINE RESERVE 141 142 MANAGEMENT PLAN Appendix 2. Native Fauna of the HIMI Marine Reserve Listed Under the EPBC Act Scientific Name Common Name Birds recorded as breeding Aptenodytes patagonicus king penguin S Catharacta lonnbergi subantarctic skua S Daption capense cape petrel S Diomeda exulans wandering albatross V S M B J A Diomeda melanophrys black–browed albatross S M B A Eudyptes chrysocome southern rockhopper penguin S Eudyptes chrysolophus macaroni penguin S Larus dominicanus kelp gull S Macronectes giganteus southern giant petrel E S M B A Oceanites oceanicus Wilson’s storm petrel S M J Pachyptila crassirostris fulmar prion S Pachyptila desolata Antarctic prion S Pelecanoides georgicus South Georgian diving petrel S Pelecanoides urinatrix common diving petrel S Phalacrocorax atriceps (e) Heard Island cormorant V S Phoebetria palpebrata light mantled sooty albatross S M B A Pygoscelis papua gentoo penguin S Sterna vittata Antarctic tern V S Non–breeding birds Catharacta maccormicki south polar skua S M J Diomedea epomophora southern royal albatross V S M B A Fregetta grallaria white–bellied storm petrel S Fregetta tropica black–bellied storm petrel S Fulmarus glacialoides southern fulmar S Garrodia nereis grey–backed storm petrel S Halobaena caerulea blue petrel V S Macronectes halli northern giant petrel V S M B A Pachyptila belcheri -

Order PROCELLARIIFORMES: Albatrosses
Text extracted from Gill B.J.; Bell, B.D.; Chambers, G.K.; Medway, D.G.; Palma, R.L.; Scofield, R.P.; Tennyson, A.J.D.; Worthy, T.H. 2010. Checklist of the birds of New Zealand, Norfolk and Macquarie Islands, and the Ross Dependency, Antarctica. 4th edition. Wellington, Te Papa Press and Ornithological Society of New Zealand. Pages 64, 78-79 & 82-83. Order PROCELLARIIFORMES: Albatrosses, Petrels, Prions and Shearwaters Checklist Committee (1990) recognised three families within the Procellariiformes, however, four families are recognised here, with the reinstatement of Pelecanoididae, following many other recent authorities (e.g. Marchant & Higgins 1990, del Hoyo et al. 1992, Viot et al. 1993, Warham 1996: 484, Nunn & Stanley 1998, Dickinson 2003, Brooke 2004, Onley & Scofield 2007). The relationships of the families within the Procellariiformes are debated (e.g. Sibley & Alquist 1990, Christidis & Boles 1994, Nunn & Stanley 1998, Livezey & Zusi 2001, Kennedy & Page 2002, Rheindt & Austin 2005), so a traditional arrangement (Jouanin & Mougin 1979, Marchant & Higgins 1990, Warham 1990, del Hoyo et al. 1992, Warham 1996: 505, Dickinson 2003, Brooke 2004) has been adopted. The taxonomic recommendations (based on molecular analysis) on the Procellariiformes of Penhallurick & Wink (2004) have been heavily criticised (Rheindt & Austin 2005) and have seldom been followed here. Family PROCELLARIIDAE Leach: Fulmars, Petrels, Prions and Shearwaters Procellariidae Leach, 1820: Eleventh room. In Synopsis Contents British Museum 17th Edition, London: 68 – Type genus Procellaria Linnaeus, 1758. Subfamilies Procellariinae and Fulmarinae and shearwater subgenera Ardenna, Thyellodroma and Puffinus (as recognised by Checklist Committee 1990) are not accepted here given the lack of agreement about to which subgenera some species should be assigned (e.g. -

Petrelsrefs V1.1.Pdf
Introduction I have endeavoured to keep typos, errors, omissions etc in this list to a minimum, however when you find more I would be grateful if you could mail the details during 2017 & 2018 to: [email protected]. Please note that this and other Reference Lists I have compiled are not exhaustive and are best employed in conjunction with other sources. Grateful thanks to Killian Mullarney and Tom Shevlin (www.irishbirds.ie) for the cover images. All images © the photographers. Joe Hobbs Index The general order of species follows the International Ornithologists' Union World Bird List (Gill, F. & Donsker, D. (eds.) 2017. IOC World Bird List. Available from: http://www.worldbirdnames.org/ [version 7.3 accessed August 2017]). Version Version 1.1 (August 2017). Cover Main image: Bulwer’s Petrel. At sea off Madeira, North Atlantic. 14th May 2012. Picture by Killian Mullarney. Vignette: Northern Fulmar. Great Saltee Island, Co. Wexford, Ireland. 5th May 2008. Picture by Tom Shevlin. Species Page No. Antarctic Petrel [Thalassoica antarctica] 12 Beck's Petrel [Pseudobulweria becki] 18 Blue Petrel [Halobaena caerulea] 15 Bulwer's Petrel [Bulweria bulweri] 24 Cape Petrel [Daption capense] 13 Fiji Petrel [Pseudobulweria macgillivrayi] 19 Fulmar [Fulmarus glacialis] 8 Giant Petrels [Macronectes giganteus & halli] 4 Grey Petrel [Procellaria cinerea] 19 Jouanin's Petrel [Bulweria fallax] 27 Kerguelen Petrel [Aphrodroma brevirostris] 16 Mascarene Petrel [Pseudobulweria aterrima] 17 Parkinson’s Petrel [Procellaria parkinsoni] 23 Southern Fulmar [Fulmarus glacialoides] 11 Spectacled Petrel [Procellaria conspicillata] 22 Snow Petrel [Pagodroma nivea] 14 Tahiti Petrel [Pseudobulweria rostrata] 18 Westland Petrel [Procellaria westlandica] 23 White-chinned Petrel [Procellaria aequinoctialis] 20 1 Relevant Publications Beaman, M. -

The Incidence, Functions and Ecological Significance of Petrel Stomach Oils
84 PROCEEDINGS OF THE NEW ZEALAND ECOLOGICAL SOCIETY, VOL. 24, 1977 THE INCIDENCE, FUNCTIONS AND ECOLOGICAL SIGNIFICANCE OF PETREL STOMACH OILS JOHN WARHAM Department of Zoology, University of Canterbury, Chrb;tchurch SUMMARY: Recent research into the origins and compositions of the stomach oils unique to sea~birds of the order Procellariifonnes is reviewed. The sources of these oils, most of which contain mainly wax esters and/or trigIycerides, is discussed in relation to the presence of such compounds in the marine environment. A number of functions are proposed as the ecological roles of the oils, including their use as slowIy~mobilisable energy and water reserves for adults and chicks and as defensive weaponry for surface-nesting species. Suggestions are made for further research, particularly into physiological and nutritional aspects. INTRODUCTION their table, a balm for their wounds, and a medicine Birds of the Order Procellariiforrnes (albatrosses, for their distempers." In New Zealand, Travers and fuJmars, shearwaters and other petrels) are peculiar Travers (1873) described how the Chatham Island in being able to store oil in their large, glandular and Morioris held young petrels over their mouths and very distensible fore-guts or proventriculi.. All petrel allowed the oil to drain directly into them. In some spedes so far examined, with the significant excep- years the St Kildans exported part of thei.r oil har~ tion of the diving petrels, Fam. Pelecanoididae, have vest, as the Australian mutton-birders stilI do with been found to contain oil at various times. The oi.1 oil from the chicks of P. tenuirostris. This has been occurs in both adults and chicks, in breeders and used as a basis for sun~tan lotions, but most nowa- non~breeders, and in birds taken at sea and on land. -

Cape Petrel (Southern)
RECOVERY OUTLINE Cape Petrel (southern) 1 Family Procellariidae 2 Scientific name Daption capense capense Linnaeus, 1758 3 Common name Cape Petrel (southern) 4 Conservation status Australian breeding population Vulnerable: D2 Population visiting Australian territory Least Concern 5 Reasons for listing The Australian population breeds at a single location (Vulnerable: D2). Globally the species is listed as Least Concern. Site fidelity is high, so it is assumed that the immigration rate is low. The national status of the breeding population is therefore determined independently of the global status (as per Gärdenfors et al., 1999). Australian breeding Estimate Reliability colonies Extent of occurrence 5,000,000 km2 low trend stable high 2 Area of occupancy 20 km medium 10 Threats trend stable low There are no imminent threats. At sea, some birds are No. of breeding birds 1,000 low likely to be caught on long-lines, but the subspecies is trend stable low usually displaced at feeding areas by larger scavengers, No. of sub-populations 1 high such as albatrosses and giant-petrels. The accidental Generation time 15 years low introduction of rats or cats is a potential threat. Global population share < 1 % high 11 Information required Level of genetic exchange low low None. 6 Infraspecific taxa D. c. australe (New Zealand region) is Least Concern. 12 Recovery objectives 12.1 Maintenance of the existing population. 7 Past range and abundance In Australian waters, breeding on Heard I. (Downes et 13 Actions completed or under way al., 1959, Woehler, 1991). Extralimitally, breeding on 13.1 Population is monitored opportunistically. islands throughout Southern Ocean. -

Appendix D New Zealand Conservation Status And
Appendix D New Zealand conservation status and International Union for Conservation of Nature (IUCN) ‘Red List’ threat classification of seabirds mentioned in the report and additionally in Paragraphs 12, 23 and 24 of my evidence. New Zealand conservation status is taken from Robertson et al. (2013), and ‘Red List’ classification from http://www.iucnredlist.org/, where further information regarding categories and criteria employed by the two systems can be found. Taxa marked * were referred to in general, non-specific terms in the report (for example, northern royal albatross here was referred to as ‘unidentified royal albatross’ in the report), but are included here as species for completeness. Taxa are listed in the order they appear in the report, then in the order they appear in Paragraphs 12, 23 and 24 of my evidence. Taxa NZ Conservation Status Red List classification Wandering albatross Migrant Vulnerable Northern royal albatross* At Risk – Naturally Uncommon Endangered Southern royal albatross* At Risk – Naturally Uncommon Vulnerable Antipodean albatross Threatened – Nationally Critical Vulnerable Gibson’s albatross Threatened – Nationally Critical Vulnerable Black-browed albatross Coloniser Near Threatened Campbell albatross At Risk - Naturally Uncommon Vulnerable Grey-headed albatross Threatened – Nationally Vulnerable Endangered Southern Buller’s albatross At Risk - Naturally Uncommon Near Threatened Salvin’s albatross Threatened – Nationally Critical Vulnerable Northern giant petrel* At Risk - Naturally Uncommon Least Concern -

Foods of the South Polar Skua Catharacta Maccormicki at Ardery Island, Windmill Islands, Antarctica
Polar Biol 2001) 24: 59±61 Ó Springer-Verlag 2001 SHORT NOTE S. C. Baker á C. Barbraud Foods of the South Polar skua Catharacta maccormicki at Ardery Island, Windmill Islands, Antarctica Accepted: 3 June 2000 Abstract South Polar skuas Catharacta maccormicki) includes clis, both steep and gentle slopes, and areas of breed on ArderyIsland in the absence of a local morainic boulder slopes. breeding population of Adelie penguins Pygoscelis In manyother Antarctic areas, feeding during the adeliae). Assessment was made of the food remains in breeding season bySouth Polar skuas, and hence their skua feeding territories in 1995/1996. The diet of South selection of breeding location, is largelydependent on Polar skuas largelyconsisted of fulmarine petrel species Adelie penguin rookeries in combination with avail- which bred on ArderyIsland. Southern fulmar Fulma- abilityof food at sea Young 1963; MuÈ ller-Schwarze and rus glacialoides) remains were the predominant prey MuÈ ller-Schwarze 1973; Trillmich 1978; Hull et al. 1994; items found, and skuas appeared to feed preferentially Norman et al. 1994). Ecklund 1961) estimated that at on this species. least 95% of skua nesting habitats in the Windmill Island group are close to Adelie penguin rookeries. The absence of breeding populations of Adelie penguins on ArderyIsland allows investigation of skua feeding where other bird species are likelyto be predominant in their Introduction diet. Other studies that have investigated the diet of skuas remote from Adelie penguin populations have ArderyIsland occurs in the Arderyand Odbert Island found food items of importance to be snow petrels SpeciallyProtected Area, Windmill Island group, near Zipan and Norman 1993), southern fulmars and CaseyStation 66 °22¢S, 110°27¢E), Antarctica. -

Long-Term Contrasted Responses to Climate of Two Antarctic Seabird Species
Ecology, 86(11), 2005, pp. 2889±2903 q 2005 by the Ecological Society of America LONG-TERM CONTRASTED RESPONSES TO CLIMATE OF TWO ANTARCTIC SEABIRD SPECIES STEPHANIE JENOUVRIER,1 CHRISTOPHE BARBRAUD, AND HENRI WEIMERSKIRCH Centre d'Etudes Biologiques de ChizeÂ, Centre National de la Recherche Scienti®que, F-79360 Villiers en Bois, France Abstract. We examined the population dynamics of two Antarctic seabirds and the in¯uence of environmental variability over a 40-year period by coupling the estimation of demographic parameters, based on capture±recapture data, and modeling, using Leslie ma- trix population models. We demonstrated that the demographic parameters showing the greatest contribution to the variance of population growth rate were adult survival for both species. Breeding success showed the same contribution as adult survival for Emperor Penguins, whereas the proportion of breeders had the next stronger contribution for Snow Petrels. The sensitivity of population growth rate to adult survival was very high and the adult survival variability was weak for both species. Snow Petrel males survived better than females, whereas Emperor Penguin males had lower survival than females. These differ- ences may be explained by the different investment in breeding. Emperor Penguin adult survival was negatively affected by air temperature during summer and winter for both sexes; male survival was negatively affected by sea ice concentration during summer, autumn, and winter. On the other hand, there was no effect of environmental covariates on Snow Petrel adult survival. The Emperor Penguin population has declined by 50% because of a decrease in adult survival related to a warming event during a regime shift in the late 1970s, whereas Snow Petrels showed their lowest numbers in 1976, but were able to skip reproduction. -
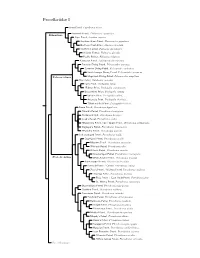
Procellariidae Species Tree
Procellariidae I Snow Petrel, Pagodroma nivea Antarctic Petrel, Thalassoica antarctica Fulmarinae Cape Petrel, Daption capense Southern Giant-Petrel, Macronectes giganteus Northern Giant-Petrel, Macronectes halli Southern Fulmar, Fulmarus glacialoides Atlantic Fulmar, Fulmarus glacialis Pacific Fulmar, Fulmarus rodgersii Kerguelen Petrel, Aphrodroma brevirostris Peruvian Diving-Petrel, Pelecanoides garnotii Common Diving-Petrel, Pelecanoides urinatrix South Georgia Diving-Petrel, Pelecanoides georgicus Pelecanoidinae Magellanic Diving-Petrel, Pelecanoides magellani Blue Petrel, Halobaena caerulea Fairy Prion, Pachyptila turtur ?Fulmar Prion, Pachyptila crassirostris Broad-billed Prion, Pachyptila vittata Salvin’s Prion, Pachyptila salvini Antarctic Prion, Pachyptila desolata ?Slender-billed Prion, Pachyptila belcheri Bonin Petrel, Pterodroma hypoleuca ?Gould’s Petrel, Pterodroma leucoptera ?Collared Petrel, Pterodroma brevipes Cook’s Petrel, Pterodroma cookii ?Masatierra Petrel / De Filippi’s Petrel, Pterodroma defilippiana Stejneger’s Petrel, Pterodroma longirostris ?Pycroft’s Petrel, Pterodroma pycrofti Soft-plumaged Petrel, Pterodroma mollis Gray-faced Petrel, Pterodroma gouldi Magenta Petrel, Pterodroma magentae ?Phoenix Petrel, Pterodroma alba Atlantic Petrel, Pterodroma incerta Great-winged Petrel, Pterodroma macroptera Pterodrominae White-headed Petrel, Pterodroma lessonii Black-capped Petrel, Pterodroma hasitata Bermuda Petrel / Cahow, Pterodroma cahow Zino’s Petrel / Madeira Petrel, Pterodroma madeira Desertas Petrel, Pterodroma -

Hawker Island, Vestfold Hills, Ingrid Christensen Coast, Princess Elizabeth Land, East Antarctica
Measure 1 (2006) Annex H Management Plan for Antarctic Specially Protected Area No. 167 HAWKER ISLAND, VESTFOLD HILLS, INGRID CHRISTENSEN COAST, PRINCESS ELIZABETH LAND, EAST ANTARCTICA 1. Description of Values to be Protected Hawker Island, lying some 300 m off the Antarctic mainland, is located 7 km south-west from the Australian Davis station in the Vestfold Hills on the Ingrid Christensen Coast, Princess Elizabeth Land, East Antarctica at 68°35’S, 77°50’E (Map A). The island supports a breeding colony of southern giant petrels (Macronectes giganteus) which is the southernmost colony of the species on continental Antarctica. The island also supports a colony of Adélie penguins and a limited number of flying birds. The southern giant petrel colony was discovered in December 1963; at that time there were 40-50 nests present, “some with eggs”. Seventeen population counts were undertaken between 1963 and 1999 (see Figure 1). A maximum of 90 nests with eggs was recorded in 1970/71. The recorded number of nests with eggs had decreased to 10 in 1983, but the two most recent surveys, conducted in 1987 and 1999, recorded 21 and 25 respectively. Southern Giant Petrels, Breeding Pairs, Hawker Island 100 90 80 70 60 50 40 Number of Pairs of Number 30 20 10 0 1960 1970 1980 1990 2000 2010 Year Figure 1: Population records for southern giant petrels (breeding pairs) at Hawker Island Hawker Island is one of only four known breeding locations for southern giant petrels on the coast of continental Antarctica. The other locations have all been designated as Antarctic Specially Protected Areas (ASPAs): ASPA No. -

Trophic Relationships Among Antarctic Fulmarine Petrels: Insights Into
MARINE ECOLOGY PROGRESS SERIES Published June 5 Mar Ecol Prog Ser I Trophic relationships among Antarctic fulmarine petrels: insights into dietary overlap and chick provisioning strategies inferred from stable-isotope (615~and 613c)analyses Peter J. Hoduml1*,Keith A. ~obson~ 'Department of Avian Sciences, University of California, Davis, California 95616-8532, USA 'canadian Wildlife Service, 115 Perimeter Road, Saskatoon, Saskatchewan S7N 0x4, Canada ABSTRACT: We used stable-isotope analysis (SIA) to evaluate trophc relationships in an Antarctic seabird community. We deterrnined natural abundances of stable-nitrogen (St5N)and stable-carbon (613C)isotopes from blood samples (n = 283) from adults and chicks of 4 Antarctic fulrnarine petrel spe- cies (Fulmarus glaciaioides, Thalassoica antarctica, Daption capense and Pagodroma nivea) during 2 consecutive breeding seasons, 1994/1995 and 1995/1996, and from representative prey items. Our objectives were to use the isotope approach to infer trophic Status and diet composition within and between species, addressing interspecific and temporal variability within this seabird community, and to investigate potential age-related differences in assumed trophic position within species. Prey 613C values ranged from -26.8% in amphipods to -23.9% in adult Antarctic silverfish. Seabird St3C values ranged from -25.3 Yw in Antarctic petrel chicks to -23.8760 in Cape petrel adults. Prey 6I5N values ranged from 4.0Yw in euphausiids to 10.7Ywin adult Antarctic silverfish. Seabird 615N values ranged from 8.47~ in Antarctic petrel adults to 12.0%0in Snow petrel chicks. There was considerable interspecific overlap in assumed trophic positions amongst the 4 petrel species, and we conclude aii species consumed fish and krill.