Standardizing Microsatellite Panels for Individual Identification of Seabirds' Snow Petrel Pagodroma Nivea and Wilson's Stor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Birdlife Australia Rarities Committee Unusual Record Report Form
BirdLife Australia Rarities Committee Unusual Record Report Form This form is intended to aid observers in the preparation of a submission for a major rarity in Australia. (It is not a mandatory requirement) Please complete all sections ensuring that you attach all relevant information including any digital images (email to [email protected] or [email protected]). Submissions to BARC should be submitted electronically wherever possible. Full Name: Rob Morris Office Use Address: Phone No: Robert P. Morris, Email: Full Name: Andrew Sutherland (first noticed the second bird) Address: Phone No: Email: Species Name: Broad-billed Prion Scientific Name: Pachyptila vittata Date(s) and time(s) of observation: 11 August 2019 First individual photographed at 12.22 – last bird photographed at 13.11. How long did you watch the bird(s)? c30+ minutes – multiple sightings of 2 birds (possibly 3) and then an additional sighting of 1 bird 20 minutes later whilst travelling, flying past and photographed. First and last date of occurrence: 11 August 2019 Distance to bird: Down to approximately 20-30 m Site Location: SE Tasmania. Approximately 42°50'36.30"S 148°24'46.23"E 22NM ENE of Pirates Bay, Eaglehawk Neck. We went north in an attempt to seek lighter winds and less swell and avoid heading straight into the strong SE winds and southerly swell. Habitat (describe habitat in which the bird was seen): Continental slope waters at a depth of approximately 260 fathoms. Sighting conditions (weather, visibility, light conditions etc.): Weather: Both days were mostly cloudy with occasional periods of bright sunshine. -

DIET and ASPECTS of FAIRY PRIONS BREEDING at SOUTH GEORGIA by P.A
DIET AND ASPECTS OF FAIRY PRIONS BREEDING AT SOUTH GEORGIA By P.A. PRINCE AND P.G. COPESTAKE ABSTRACT A subantarctic population of the Fairy Prion (Pachyprzla turtur) was studied at South Georgia in 1982-83. Full measurements of breeding birds are given, together with details of breeding habitat, the timing of the main breeding cycle events, and chick growth (weight and wing, culmen and tarsus length). Regurgitated food samples showed the diet to be mainly Crustacea (96% by weight), fish and squid comprising the rest. Of crustaceans, Antarctic krill made up 38% of items and 80% by weight. Copepods (four species, mostly Rhincalanus gigas) made up 39% of items but only 4% by weight; amphipods [three species, principally Themisto gaudichaudii made up 22% of items and 16% by weight. Diet and frequency of chick feeding are compared with those of Antarctic Prions and Blue Petrels at the same site; Fairy Prions are essentially intermediate. INTRODUCTION The Fairy Prion (Pachyptila turtur) is one of six members of a genus confined to the temperate and subantarctic regions of the Southern Hemisphere. With the Fulmar Prion (P. crassirostris), it forms the subgenus Pseudoprion. Its main area of breeding distribution is between the Antarctic Polar Front and the Subtropical Convergence. It is widespread in the New Zealand region, from the north of the North Island south to the Antipodes Islands and Macquarie Island, where only about 40 pairs survive (Brothers 1984). Although widespread in the Indian Ocean at the Prince Edward, Crozet and Kerguelen Islands, in the South Atlantic Ocean it is known to breed only on Beauchene Island (Falkland Islands) (Strange 1968, Smith & Prince 1985) and South Georgia (Prince & Croxall 1983). -

Management Plan for Antarctic Specially Protected Area No
Measure 2 (2005) Annex E Management Plan for Antarctic Specially Protected Area No. 120 POINTE-GÉOLOGIE ARCHIPELAGO, TERRE ADÉLIE Jean Rostand, Le Mauguen (former Alexis Carrel), Lamarck and Claude Bernard Islands, The Good Doctor’s Nunatak and breeding site of Emperor Penguins 1. Description of Values to be Protected In 1995, four islands, a nunatak and a breeding ground for emperor penguins were classified as an Antarctic Specially Protected Area (Measure 3 (1995), XIX ATCM, Seoul) because they were a representative example of terrestrial Antarctic ecosystems from a biological, geological and aesthetics perspective. A species of marine mammal, the Weddell seal (Leptonychotes weddelli) and various species of birds breed in the area: emperor penguin (Aptenodytes forsteri); Antarctic skua (Catharacta maccormicki); Adélie penguins (Pygoscelis adeliae); Wilson’s petrel (Oceanites oceanicus); giant petrel (Macronectes giganteus); snow petrel (Pagodrama nivea), cape petrel (Daption capense). Well-marked hills display asymmetrical transverse profiles with gently dipping northern slopes compared to the steeper southern ones. The terrain is affected by numerous cracks and fractures leading to very rough surfaces. The basement rocks consist mainly of sillimanite, cordierite and garnet-rich gneisses which are intruded by abundant dikes of pink anatexites. The lowest parts of the islands are covered by morainic boulders with a heterogenous granulometry (from a few cm to more than a m across). Long-term research and monitoring programs of birds and marine mammals have been going on for a long time already (since 1952 or 1964 according to the species). A database implemented in 1981 is directed by the Centre d'Etudes Biologiques de Chize (CEBC-CNRS). -

Part 4 Appendices
Part 4 Appendices HEARD ISLAND AND MCDONALD ISLANDS MARINE RESERVE 139 Appendix 1. Proclamation of Heard Island and McDonald Islands Marine Reserve 140 MANAGEMENT PLAN HEARD ISLAND AND MCDONALD ISLANDS MARINE RESERVE 141 142 MANAGEMENT PLAN Appendix 2. Native Fauna of the HIMI Marine Reserve Listed Under the EPBC Act Scientific Name Common Name Birds recorded as breeding Aptenodytes patagonicus king penguin S Catharacta lonnbergi subantarctic skua S Daption capense cape petrel S Diomeda exulans wandering albatross V S M B J A Diomeda melanophrys black–browed albatross S M B A Eudyptes chrysocome southern rockhopper penguin S Eudyptes chrysolophus macaroni penguin S Larus dominicanus kelp gull S Macronectes giganteus southern giant petrel E S M B A Oceanites oceanicus Wilson’s storm petrel S M J Pachyptila crassirostris fulmar prion S Pachyptila desolata Antarctic prion S Pelecanoides georgicus South Georgian diving petrel S Pelecanoides urinatrix common diving petrel S Phalacrocorax atriceps (e) Heard Island cormorant V S Phoebetria palpebrata light mantled sooty albatross S M B A Pygoscelis papua gentoo penguin S Sterna vittata Antarctic tern V S Non–breeding birds Catharacta maccormicki south polar skua S M J Diomedea epomophora southern royal albatross V S M B A Fregetta grallaria white–bellied storm petrel S Fregetta tropica black–bellied storm petrel S Fulmarus glacialoides southern fulmar S Garrodia nereis grey–backed storm petrel S Halobaena caerulea blue petrel V S Macronectes halli northern giant petrel V S M B A Pachyptila belcheri -

Spatial Segregation of Prions and Blue Petrels Is Explained by Differences in Preferred Sea Surface Temper
Animal behaviour Cool, cold or colder? Spatial segregation rsbl.royalsocietypublishing.org of prions and blue petrels is explained by differences in preferred sea surface temperatures Research 1 2 2 2 Cite this article: Quillfeldt P, Cherel Y, Delord Petra Quillfeldt , Yves Cherel , Karine Delord and Henri Weimerkirch K, Weimerkirch H. 2015 Cool, cold or colder? 1Department of Animal Ecology and Systematics, Justus-Liebig-Universita¨t Giessen, 35392 Giessen, Germany Spatial segregation of prions and blue petrels 2Centre d’Etudes Biologiques de Chize´, UMR 7372 CNRS-Universite´ de La Rochelle, 79360 Villiers-en-Bois, France is explained by differences in preferred sea surface temperatures. Biol. Lett. 11: 20141090. The Southern Ocean provides one of the largest environmental gradients on http://dx.doi.org/10.1098/rsbl.2014.1090 Earth that lacks geographical barriers, and small but highly mobile petrels living there may offer fine models of evolution of diversity along environmental gradients. Using geolocation devices, we investigated the winter distribution of closely related petrel species breeding sympatrically in the southern Indian Received: 31 December 2014 Ocean, and applied ecological niche models to compare environmental con- ditions in the habitat used. We show that thin-billed prions (Pachyptila Accepted: 27 March 2015 belcheri), Antarctic prions (Pachyptila desolata) and blue petrels (Halobaena caerulea) from the Kerguelen archipelago in the southern Indian Ocean segre- gate latitudinally, sea surface temperature being the most important variable separating the distribution of the species. Antarctic prions spent the winter Subject Areas: north of the Polar Front in temperate waters, whereas blue petrels were ecology, behaviour found south of the Polar Front in Antarctic waters. -

Petrelsrefs V1.1.Pdf
Introduction I have endeavoured to keep typos, errors, omissions etc in this list to a minimum, however when you find more I would be grateful if you could mail the details during 2017 & 2018 to: [email protected]. Please note that this and other Reference Lists I have compiled are not exhaustive and are best employed in conjunction with other sources. Grateful thanks to Killian Mullarney and Tom Shevlin (www.irishbirds.ie) for the cover images. All images © the photographers. Joe Hobbs Index The general order of species follows the International Ornithologists' Union World Bird List (Gill, F. & Donsker, D. (eds.) 2017. IOC World Bird List. Available from: http://www.worldbirdnames.org/ [version 7.3 accessed August 2017]). Version Version 1.1 (August 2017). Cover Main image: Bulwer’s Petrel. At sea off Madeira, North Atlantic. 14th May 2012. Picture by Killian Mullarney. Vignette: Northern Fulmar. Great Saltee Island, Co. Wexford, Ireland. 5th May 2008. Picture by Tom Shevlin. Species Page No. Antarctic Petrel [Thalassoica antarctica] 12 Beck's Petrel [Pseudobulweria becki] 18 Blue Petrel [Halobaena caerulea] 15 Bulwer's Petrel [Bulweria bulweri] 24 Cape Petrel [Daption capense] 13 Fiji Petrel [Pseudobulweria macgillivrayi] 19 Fulmar [Fulmarus glacialis] 8 Giant Petrels [Macronectes giganteus & halli] 4 Grey Petrel [Procellaria cinerea] 19 Jouanin's Petrel [Bulweria fallax] 27 Kerguelen Petrel [Aphrodroma brevirostris] 16 Mascarene Petrel [Pseudobulweria aterrima] 17 Parkinson’s Petrel [Procellaria parkinsoni] 23 Southern Fulmar [Fulmarus glacialoides] 11 Spectacled Petrel [Procellaria conspicillata] 22 Snow Petrel [Pagodroma nivea] 14 Tahiti Petrel [Pseudobulweria rostrata] 18 Westland Petrel [Procellaria westlandica] 23 White-chinned Petrel [Procellaria aequinoctialis] 20 1 Relevant Publications Beaman, M. -

Master Wildlife List-Peninsula
Antarctic Peninsula Expedition Wildlife List Date Family Species Twitcher List 8-Mar 9-Mar 10-Mar 11-Mar 12-Mar 13-Mar 14-Mar 15-Mar 16-Mar 17-Mar Adelie X X Chinstrap X X X X X X Emperor Penguins Gentoo X X X X X X X Macaroni X X Magellanic X X Unidentifed X X X Black-browed X X X X X Grey-headed X X X X Light-mantled Sooty X X Albatross Northern Royal X X X Southern Royal X X X Wandering X X X Northern X X X X Southern X X X X X X X Giant Petrel Southern (white morph) X X X Unidentified Soft-plumaged Petrel X X X X Gadfly Petrels Soft-plumaged Petrel (Dark) X X Antarctic Petrel Cape Petrel X X X X X X Snow Petrel ? ? Petrels Southern Fulmar X X X X White-chinned Petrel X X X Grey Petrel Great Shearwater X X Shearwaters Sooty Shearwater X X X X Blue Petrel Antarctic Prion X X X X BIRDS Blue Petrels & Prions Fairy Prion Slender-billed Prion X X Unidentified Black-bellied X X X X Grey-backed Storm-petrels Wilson’s X X X X X X X X Unidentified Common Diving-petrels Magellanic Unidentified X X X Antarctic Shag X X X X X X Shags Imperial Shag X X Rock Shag X X Chilean Skua X X South Polar Skua X X X X X X Skuas Subantarctic Skua X X X X X X Unidentified X X Brown-hooded Gull X X Gulls Dolphin Gull X X Kelp Gull X X X X X X X Antarctic X X X X X Terns Arctic South American Blk-crowned Night Heron Blackish Oystercatcher Shorebirds Magellanic Oystercatcher Magellanic Snipe Pale-faced Sheathbill X X X X Other Arnoux's Beaked Blue Cuvier's Beaked Fin Whale Hector's Beaked Humpback X X X Long-finned Pilot Whales Minke X X X X Sei X X X X Southern Bottlenose Southern Right Sperm Beaked (sp.) X X MAMMALS Unidentified Dusky Hourglass X X Orca (Killer Whale) X X Dolphins Peale’s X X Other Unidentified Crabeater X X X X X Leopard X X X X Phocids Southern Elephant X X X Weddell X X X X Antarctic Fur Seal X X X X X Otariids South American Fur Seal South American Sealion OTHER Chilean swallow X X Salp X 8-Mar 9-Mar 10-Mar 11-Mar 12-Mar 13-Mar 14-Mar 15-Mar 16-Mar 17-Mar Twitcher List Date www.oneoceanexpedi,ons.com. -

Importance of Ice Algal Production for Top Predators: New Insights Using Sea-Ice Biomarkers
Vol. 513: 269–275, 2014 MARINE ECOLOGY PROGRESS SERIES Published October 22 doi: 10.3354/meps10971 Mar Ecol Prog Ser FREEREE ACCESSCCESS Importance of ice algal production for top predators: new insights using sea-ice biomarkers A. Goutte1,2,*, J.-B. Charrassin1, Y. Cherel2, A. Carravieri2, S. De Grissac2, G. Massé1,3 1LOCEAN/IPSL — UMR 7159 Centre National de la Recherche Scientifique/Université Pierre et Marie Curie/ Institut de Recherche pour le Développement/Museum National d’Histoire Naturelle, 75005 Paris, France 2Centre d’Etudes Biologiques de Chizé, Centre National de la Recherche Scientifique, UPR 1934, 79360 Beauvoir sur Niort, France 3Centre National de la Recherche Scientifique and Université Laval, UMI 3376, Takuvik, Québec G1V 0A6, Canada ABSTRACT: Antarctic seals and seabirds are strongly dependent on sea-ice cover to complete their life history. In polar ecosystems, sea ice provides a habitat for ice-associated diatoms that en - sures a substantial production of organic matter. Recent studies have presented the potential of highly branched isoprenoids (HBIs) for tracing carbon flows from ice algae to higher-trophic-level organisms. However, to our knowledge, this new method has never been applied to sub-Antarctic species and Antarctic seals. Moreover, seasonal variations in HBI levels have never been investi- gated in Antarctic predators, despite a likely shift in food source from ice-derived to pelagic organic matter after sea-ice retreat. In the present study, we described HBI levels in a community of seabirds and seals breeding in Adélie Land, Antarctica. We then validated that sub-Antarctic seabirds had lower levels of diene, a HBI of sea-ice diatom origin, and higher levels of triene, a HBI of phytoplanktonic origin, compared with Antarctic seabirds. -

Densities of Antarctic Seabirds at Sea and the Presence of the Krill Eupha Usia Superba
DENSITIES OF ANTARCTIC SEABIRDS AT SEA AND THE PRESENCE OF THE KRILL EUPHA USIA SUPERBA BRYAN S. OBST Departmentof Biology,University of California,Los Angeles, California 90024 USA ABSTRACT.--Theantarctic krill Euphausiasuperba forms abundant,well-organized schools in the watersoff the AntarcticPeninsula. Mean avian densityis 2.6 timesgreater in waters where krill schoolsare present than in waters without krill schools.Seabird density is a good predictorof the presenceof krill. Seabirddensity did not correlatewith krill density or krill schooldepth. Disoriented krill routinely were observedswimming near the surface above submergedschools, providing potential prey for surface-feedingbirds. Responsesof seabird speciesto the distribution of krill schoolsvaried. The small to me- dium-sizeprocellariiform species were the best indicatorsof krill schools;large procellari- iforms and coastalspecies were poor indicators.Pygoscelis penguins occurredat high den- sitiesonly in the presenceof krill schools.These responses are consistentwith the constraints imposedby the metabolicrequirements and reproductivestrategies of eachof thesegroups. Krill schoolswere detectednear the seasurface throughout the day. Correlationsbetween seabirddensity and the presenceof krill during daylight hourssuggest that diurnal foraging is important to the seabirdsof this region. Received19 December1983, accepted4 December 1984. RELATIVELY little is known about the factors birds depend on directly or indirectly for food influencing the distribution of seabirdsin the (Haury et al. 1978). These observationssuggest marine habitat. The past decade has produced that relatively small-scalephenomena, such as a number of studiesattempting to correlatepat- local concentrationsof prey, may be of major terns of avian abundance and distribution with importance in determining the patterns of sea- physical featuresof the oceansuch as currents bird distribution within the broad limits set by and convergences,water masses,and temper- featuresof the physical ocean. -

Foods of the South Polar Skua Catharacta Maccormicki at Ardery Island, Windmill Islands, Antarctica
Polar Biol 2001) 24: 59±61 Ó Springer-Verlag 2001 SHORT NOTE S. C. Baker á C. Barbraud Foods of the South Polar skua Catharacta maccormicki at Ardery Island, Windmill Islands, Antarctica Accepted: 3 June 2000 Abstract South Polar skuas Catharacta maccormicki) includes clis, both steep and gentle slopes, and areas of breed on ArderyIsland in the absence of a local morainic boulder slopes. breeding population of Adelie penguins Pygoscelis In manyother Antarctic areas, feeding during the adeliae). Assessment was made of the food remains in breeding season bySouth Polar skuas, and hence their skua feeding territories in 1995/1996. The diet of South selection of breeding location, is largelydependent on Polar skuas largelyconsisted of fulmarine petrel species Adelie penguin rookeries in combination with avail- which bred on ArderyIsland. Southern fulmar Fulma- abilityof food at sea Young 1963; MuÈ ller-Schwarze and rus glacialoides) remains were the predominant prey MuÈ ller-Schwarze 1973; Trillmich 1978; Hull et al. 1994; items found, and skuas appeared to feed preferentially Norman et al. 1994). Ecklund 1961) estimated that at on this species. least 95% of skua nesting habitats in the Windmill Island group are close to Adelie penguin rookeries. The absence of breeding populations of Adelie penguins on ArderyIsland allows investigation of skua feeding where other bird species are likelyto be predominant in their Introduction diet. Other studies that have investigated the diet of skuas remote from Adelie penguin populations have ArderyIsland occurs in the Arderyand Odbert Island found food items of importance to be snow petrels SpeciallyProtected Area, Windmill Island group, near Zipan and Norman 1993), southern fulmars and CaseyStation 66 °22¢S, 110°27¢E), Antarctica. -

Long-Term Contrasted Responses to Climate of Two Antarctic Seabird Species
Ecology, 86(11), 2005, pp. 2889±2903 q 2005 by the Ecological Society of America LONG-TERM CONTRASTED RESPONSES TO CLIMATE OF TWO ANTARCTIC SEABIRD SPECIES STEPHANIE JENOUVRIER,1 CHRISTOPHE BARBRAUD, AND HENRI WEIMERSKIRCH Centre d'Etudes Biologiques de ChizeÂ, Centre National de la Recherche Scienti®que, F-79360 Villiers en Bois, France Abstract. We examined the population dynamics of two Antarctic seabirds and the in¯uence of environmental variability over a 40-year period by coupling the estimation of demographic parameters, based on capture±recapture data, and modeling, using Leslie ma- trix population models. We demonstrated that the demographic parameters showing the greatest contribution to the variance of population growth rate were adult survival for both species. Breeding success showed the same contribution as adult survival for Emperor Penguins, whereas the proportion of breeders had the next stronger contribution for Snow Petrels. The sensitivity of population growth rate to adult survival was very high and the adult survival variability was weak for both species. Snow Petrel males survived better than females, whereas Emperor Penguin males had lower survival than females. These differ- ences may be explained by the different investment in breeding. Emperor Penguin adult survival was negatively affected by air temperature during summer and winter for both sexes; male survival was negatively affected by sea ice concentration during summer, autumn, and winter. On the other hand, there was no effect of environmental covariates on Snow Petrel adult survival. The Emperor Penguin population has declined by 50% because of a decrease in adult survival related to a warming event during a regime shift in the late 1970s, whereas Snow Petrels showed their lowest numbers in 1976, but were able to skip reproduction. -
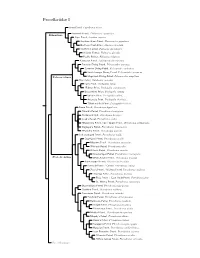
Procellariidae Species Tree
Procellariidae I Snow Petrel, Pagodroma nivea Antarctic Petrel, Thalassoica antarctica Fulmarinae Cape Petrel, Daption capense Southern Giant-Petrel, Macronectes giganteus Northern Giant-Petrel, Macronectes halli Southern Fulmar, Fulmarus glacialoides Atlantic Fulmar, Fulmarus glacialis Pacific Fulmar, Fulmarus rodgersii Kerguelen Petrel, Aphrodroma brevirostris Peruvian Diving-Petrel, Pelecanoides garnotii Common Diving-Petrel, Pelecanoides urinatrix South Georgia Diving-Petrel, Pelecanoides georgicus Pelecanoidinae Magellanic Diving-Petrel, Pelecanoides magellani Blue Petrel, Halobaena caerulea Fairy Prion, Pachyptila turtur ?Fulmar Prion, Pachyptila crassirostris Broad-billed Prion, Pachyptila vittata Salvin’s Prion, Pachyptila salvini Antarctic Prion, Pachyptila desolata ?Slender-billed Prion, Pachyptila belcheri Bonin Petrel, Pterodroma hypoleuca ?Gould’s Petrel, Pterodroma leucoptera ?Collared Petrel, Pterodroma brevipes Cook’s Petrel, Pterodroma cookii ?Masatierra Petrel / De Filippi’s Petrel, Pterodroma defilippiana Stejneger’s Petrel, Pterodroma longirostris ?Pycroft’s Petrel, Pterodroma pycrofti Soft-plumaged Petrel, Pterodroma mollis Gray-faced Petrel, Pterodroma gouldi Magenta Petrel, Pterodroma magentae ?Phoenix Petrel, Pterodroma alba Atlantic Petrel, Pterodroma incerta Great-winged Petrel, Pterodroma macroptera Pterodrominae White-headed Petrel, Pterodroma lessonii Black-capped Petrel, Pterodroma hasitata Bermuda Petrel / Cahow, Pterodroma cahow Zino’s Petrel / Madeira Petrel, Pterodroma madeira Desertas Petrel, Pterodroma