Colour Vision in Marine Organisms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Training Manual Series No.15/2018
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CMFRI Digital Repository DBTR-H D Indian Council of Agricultural Research Ministry of Science and Technology Central Marine Fisheries Research Institute Department of Biotechnology CMFRI Training Manual Series No.15/2018 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual This is a limited edition of the CMFRI Training Manual provided to participants of the “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals” organized by the Marine Biotechnology Division of Central Marine Fisheries Research Institute (CMFRI), from 2nd February 2015 - 31st March 2018. Principal Investigator Dr. P. Vijayagopal Compiled & Edited by Dr. P. Vijayagopal Dr. Reynold Peter Assisted by Aditya Prabhakar Swetha Dhamodharan P V ISBN 978-93-82263-24-1 CMFRI Training Manual Series No.15/2018 Published by Dr A Gopalakrishnan Director, Central Marine Fisheries Research Institute (ICAR-CMFRI) Central Marine Fisheries Research Institute PB.No:1603, Ernakulam North P.O, Kochi-682018, India. 2 Foreword Central Marine Fisheries Research Institute (CMFRI), Kochi along with CIFE, Mumbai and CIFA, Bhubaneswar within the Indian Council of Agricultural Research (ICAR) and Department of Biotechnology of Government of India organized a series of training programs entitled “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals”. -

Saltwater Aquariums for Dummies‰
01_068051 ffirs.qxp 11/21/06 12:02 AM Page iii Saltwater Aquariums FOR DUMmIES‰ 2ND EDITION by Gregory Skomal, PhD 01_068051 ffirs.qxp 11/21/06 12:02 AM Page ii 01_068051 ffirs.qxp 11/21/06 12:02 AM Page i Saltwater Aquariums FOR DUMmIES‰ 2ND EDITION 01_068051 ffirs.qxp 11/21/06 12:02 AM Page ii 01_068051 ffirs.qxp 11/21/06 12:02 AM Page iii Saltwater Aquariums FOR DUMmIES‰ 2ND EDITION by Gregory Skomal, PhD 01_068051 ffirs.qxp 11/21/06 12:02 AM Page iv Saltwater Aquariums For Dummies®, 2nd Edition Published by Wiley Publishing, Inc. 111 River St. Hoboken, NJ 07030-5774 www.wiley.com Copyright © 2007 by Wiley Publishing, Inc., Indianapolis, Indiana Published by Wiley Publishing, Inc., Indianapolis, Indiana Published simultaneously in Canada No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning, or otherwise, except as permit- ted under Sections 107 or 108 of the 1976 United States Copyright Act, without either the prior written permission of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, 978-750-8400, fax 978-646-8600. Requests to the Publisher for permission should be addressed to the Legal Department, Wiley Publishing, Inc., 10475 Crosspoint Blvd., Indianapolis, IN 46256, 317-572-3447, fax 317-572-4355, or online at http://www.wiley.com/go/permissions. Trademarks: Wiley, the Wiley Publishing logo, For Dummies, the Dummies Man logo, A Reference for the Rest of Us!, The Dummies Way, Dummies Daily, The Fun and Easy Way, Dummies.com, and related trade dress are trademarks or registered trademarks of John Wiley & Sons, Inc., and/or its affiliates in the United States and other countries, and may not be used without written permission. -

Science News. Click OR Call 1-800-552-4412
Hide and See: Science News Online, Nov. 6, 2004 Subscribe to Science News. Click OR call 1-800-552-4412. Week of Nov. 6, 2004; Vol. 166, No. 19 , p. 296 Hide and See Subscribe to Science News in Conflicting views of reef-fish colors an audio format. Susan Milius A lot of things can be said about a shirt that sports images of coral-reef fish, but "subtly colored" isn't one of them. Oddly enough, that characterization does get used when biologists talk about the reef creatures. Although the fish may dazzle the human eye with scarlet, rose, yellow, turquoise, Login problems? emerald, and dozens of other shades, some theorists have proposed that, Science News Books. Click here. in the complexity of a reef, the riot of fish colors serves as camouflage. a5511_1760.jpg POPULAR HUES. Calculations based Math Trek Science News e-LETTER. Football's Overtime Bias on fish vision suggest that from a distance yellows Food for Thought blend in with a Pesticide Disposal Goes generic reef Green background, and the blues fade into vistas of water. A Science Safari fish among Shop at the Science Mall. Bat Moves and More branching corals would be hidden TimeLine like a soldier 70 Years Ago in Science wearing camouflage. News PhotoDisc Then again, some scientists have suggested the opposite notion—that brilliant colors send big, bold messages that may be come-ons or warnings or both. People can theorize till the cowfish come home about what they see on a reef, but what matters is what fish see, and that's been hard to determine. -
An Analysis of the West Nggela (Solomon Islands) Fish Taxonomy
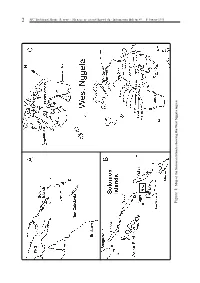
2 SPC Traditional Marine Resource Management and Knowledge Information Bulletin #9 – February 1998 Map of the Solomon Islands showing West Nggela region Figure 1: Figure SPC Traditional Marine Resource Management and Knowledge Information Bulletin #9 – February 1998 3 What’s in a name? An analysis of the West Nggela (Solomon Islands) fish taxonomy. by Simon Foale 1 Introduction Lobotidae, Gerreidae, Sparidae, Ephippidae, Chaetodontidae, Pomacentridae, Cirhitidae, Accurate knowledge about the behaviour, biol- Polynemidae, Labridae, Opistognathidae, ogy and ecology of organisms comprising marine Trichonotidae, Pinguipedidae, Blenniidae, fisheries is a vital prerequisite for their manage- Gobiidae, Microdesmidae, Zanclidae, Bothidae, ment. Before beginning any study on local knowl- Pleuronectidae, and Soleidae. edge of marine fauna, a working knowledge of The English names of many species of fish vary their local names must be obtained. Moreover, a quite a bit, even within one country such as great deal of local knowledge can often emerge in Australia. For most of the species listed in the very process of obtaining names (Ruddle, Appendix 1, I have used the English names given 1994). A detailed treatment of the local naming by Randall et al. (1990). For species not included in system of West Nggela marine fauna is given in Randall et al. (1990), names from Kailola (1987a, b, this paper. 1991) were used. Methods Results Local names of fish were collected by asking Appendix 1 contains 350 unique Nggela folk people to provide the Nggela names for fishes taxa for cartilaginous and bony fishes, together from photographs in books featuring most of the with the scientific (Linnean) taxa they correspond common Indo-Pacific species (Randall et al., 1990 to and, where available, a brief note describing an and Myers, 1991). -

Print Article
Print Article http://www.sciencenews.org/scripts/printthis.asp?clip=%2Farticles... Print Article Close Window Science News Online Week of Nov. 6, 2004; Vol. 166, No. 19 Hide and See Conflicting views of reef-fish colors Susan Milius A lot of things can be said about a shirt that sports images of coral-reef fish, but "subtly colored" isn't one of them. Oddly enough, that characterization does get used when biologists talk about the reef creatures. Although the fish may dazzle the human eye with scarlet, rose, yellow, turquoise, emerald, and dozens of other shades, some theorists have proposed that, in the complexity of a reef, the riot of fish colors serves as camouflage. POPULAR HUES. Calculations based on fish vision suggest that from a distance yellows blend in with a generic reef background, and the blues fade into vistas of water. A fish among branching corals would be hidden like a soldier wearing camouflage. PhotoDisc Then again, some scientists have suggested the opposite notion—that brilliant colors send big, bold messages that may be come-ons or warnings or both. People can theorize till the cowfish come home about what they see on a reef, but what matters is what fish see, and that's been hard to determine. Improvements in cameras and in equipment for analyzing light and color are now inspiring new approaches to approximating a fish-eye view of the reefs. Looking at the abundant coloration from a fishy perspective, the new work demonstrates that people can be quite wrong about what's showy and what's subtle. -

The Fish Communities and Main Fish Populations of the Jurien Bay Marine Park
The fish communities and main fish populations of the Jurien Bay Marine Park Fairclough, D.V., Potter, I.C., Lek, E., Bivoltsis, A.K. and Babcock, R.C. Strategic Research Fund for the Marine Environment Collaborative Research Project Final Report May 2011 2 The fish communities and main fish populations of the Jurien Bay Marine Park Fairclough, D.V. Potter, I.C. Lek, E. Bivoltsis, A.K. Babcock, R.C. May 2011 Centre for Fish and Fisheries Research Murdoch University, South Street, Murdoch Western Australia 6150 ISBN: 978-0-86905-999-9 This work is copyright. Except as permitted under the Copyright Act 1968 (Cth), no part of this publication may be reproduced by any process, electronic or otherwise, without the specific written permission of the copyright owners. Neither may information be stored electronically in any form whatsoever without such permission. 3 4 Table of Contents 1.0 Executive Summary...........................................................................................................v 2.0 Acknowledgements ..........................................................................................................vii 3.0 General Introduction.........................................................................................................1 3.1 Marine protected areas.....................................................................................................1 3.1.1 Fisheries management goals .....................................................................................1 3.1.2 Indirect effects of MPAs...........................................................................................2 -

An Examination of Marine Communities Living on The
pI ene d...e./ v" 1"1 7 (). 4f?P~At<.TNG. '7 t-t-A ~ I . ~ Y\ ,.,..pr€ 1t'A lY N 1'"'\ ()~ e .z.. p £;<-2,., GEOMARINE PTY. LTD. AN EXAMINATION OF MARINE COMMUNITIES LIVING ON THE NORTHERN SIDE OF LENNOX HEAD IN RELATION TO PROPOSED FORESHORE RESTORATION November 1989 by J.H. LAXTON and J. DUELL. " , J.H. & E.S. LAXTON - ENVIRONMENTAL CONSULTANTS PIL 170 WARRIMOO AVE., ST IVES, SYDNEY, N.S.W. 2075. AUSTRALIA. Repair, ro-e.lign, Construct a small sand Ropalr, re-align and Construct boulder wall ZCI "'T1 raise crest of bouldor with crost e.ligned to om stronglhon and ralso rotainlng groyne to act :o-i G') crost of bolidor wall as a boatmmp e.lso. wall along bock of seaward bolX1dary of -i:!:; C along the back of the bonch. properties and fill be :I:r ::xl boach whoro required. Raise the lovol of the hind wall te roclaim oC/) properties. m ramp at the back of the Nourish bench with , ,,0 ...... Nourish bonch with boach to provent wavo 10,000 cubic motres of r" Construct a sand rotalnlng sand scraped off tho Estobllsh ~unding sot m-i groyno 100m long as an 20,000 cubic motres of overtopping during sovore storms. berm north of the ffl back line, minimum Z:I: oxtonslon of lano. sand scmped off the zm borm north of Lnke noss camp and bury flocr lovols and ap o-u Nnsworth and bury Incorpomto tho storm wall undor duno. proprlato foundation X:o Fill tho two properties on conditions. -

Biochemical Properties of Anemone Fish Mucous to Understand Its Adaptation to Sea Anemone
BIOCHEMICAL PROPERTIES OF ANEMONE FISH MUCOUS TO UNDERSTAND ITS ADAPTATION TO SEA ANEMONE BY NAJATUL SU AD BINTI ABDULLAH A thesis submitted in fulfilment of the requirement for the degree of Master of Science (Biosciences) Kulliyyah of Science International Islamic University Malaysia JANUARY 2016 ABSTRACT The study of anemone fish mucous to understand anemone fish adaptation to sea anemone was aimed to identify the biochemical composition in the fish mucous that enabled the protection of anemone fish against the stinging tentacles of sea anemones. Upon sampling, one species of sea anemone, Heteractis magnifica was identified with its resident anemone fish, the false clownfish, Amphiprion ocellaris, along with 2 other non-symbiont coral reef fish species, the scissor-tailed sergeant, Abudefduf sexfasciatus (Family Pomacentridae) and the moon wrasse, Thalassoma lunare (Family Labridae) to provide comparison and knowledge insight into anemone fish ability to live unharmed along the tentacles of sea anemone, H. magnifica. The scissor-tailed sergeant and the moon wrasse are among many other coral reef fishes that are observed to be stung upon contact with the sea anemone tentacles that elicit nematocyst discharge. Mucous of false clownfish, A. ocellaris was extracted and investigated for its glycoprotein by studying the protein and sialic acid content and compared against the mucous content of sea anemone, H. magnifica, and other fish species, the scissor-tailed sergeant, A. sexfasciatus and the moon wrasse, T. lunare. Protein identification was done by SDS-PAGE and MALDI-TOF analysis while thiobarbituric acid assay followed by HPLC detection were performed to assess sialic acid content in the mucous. -

Coral Reef Fish Community at Pulau Bidong, Terengganu, South China Sea
Journal of Sustainability Science and Management eISSN: 2672-7226 Volume 16 Number 5, July 2021: 48-66 © Penerbit UMT CORAL REEF FISH COMMUNITY AT PULAU BIDONG, TERENGGANU, SOUTH CHINA SEA MUHAMMAD AMINUDIN AFIQ-FIRDAUS1, CHUIN-SIEW LIM1, MOHD SAFUAN CHE DIN1, SITI TAFZILMERIAM SHEIKH ABDUL KADIR1, AZWARINA MOHD AZMI RAMASAMY2, NORATIKAH AB MANAF2, MOHD SHAROL ALI3, LIAM LACHS1,4, HONOR E. WRIGHT5 AND ZAINUDIN BACHOK1* 1Institute of Oceanography and Environment (INOS), 2South China Sea Repository & Reference Centre (RRC) Institute of Oceanography and Environment, 3Faculty of Fisheries and Food Sciences (FPSM), Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia. 4Marine Biology, Ecology and Biodiversity, Vrije Universiteit Brussel (VUB), Belgium. 5The School of Energy, Geoscience, Infrastructure and Society (EGIS), Heriot-Watt University, Edinburgh, Scotland, UK. *Corresponding author: [email protected] Submitted final draft: 13 July 2020 Accepted: 23 September 2020 http://doi.org/10.46754/jssm.2021.07.004 Abstract: Reef fishes are among the main constituents of coral reef ecosystems and an indicator of ecosystem health. The aim of this study is to determine the species occurrences of coral reef fish at Pulau Bidong, which is in the South China Sea off the east coast state of Terengganu, Peninsular Malaysia. Fish surveys were conducted from May to July 2018 using the visual census technique during SCUBA dives around the island. A total of 30 families, 61 genera and 101 species of coral reef fishes were identified. This indicates considerably high fish diversity and reflects the good condition of the reefs. Of the species identified, 65 % were reef-associated, 12 % were pelagic-oceanic and the rest (23 %) appeared to be found in both zones. -

A Lexicographical Introduction and Inventory of Pendau Fish Names*
A Lexicographical Introduction and Inventory of Pendau * Fish Names Phil Quick SIL International [email protected] ABSTRACT This paper introduces the first inventory of fish names in Pendau (Tolitoli language group in Central Sulawesi, Indonesia). Among other purposes this paper provides a documentation of about 290 Pendau fish names (in an appended dictionary format), provides other Sulawesi specialists with comparative data, provides field linguists with an outline of some of the issues involved in descriptive documentation of flora and fauna, and provides new data for historical and comparative linguists. Topics covered in this paper are presented in such a way as to help researchers evaluate either the entire inventory of fish names or particular entries of interest. 1. INTRODUCTION This paper introduces the first inventory of fish names in Pendau (Tomini-Tolitoli language group in Central Sulawesi, Indonesia). The purpose of this portion of the paper is to focus on identifying fish names and the total number of fish names possible in a language, understanding the syntax used for binomial names, understanding some of the innovations and folk taxonomy employed, highlight those fish names that begin with one of the two formatives si and ’ali, as in sinobulung ‘various parrotfish species’ and ’alibambang ‘various butterflyfish species’, various bannerfish species, and various angelfish species’, and finally discuss the potential for discovering new species. The identification of fish names and other flora and fauna in a language is not a simple task. This paper also reviews the problems and challenges involved in providing a quality inventory that can be used by other researchers. -

Cleaning Interactions by Bluestreak Cleaner Wrasse (Labroides Dimidiatus) and Moon Wrasse (Thalassoma Lunare) on Pelagic Thesher Sharks (Alopias Pelagicus)
Cleaning interactions by bluestreak cleaner wrasse (Labroides dimidiatus) and moon wrasse (Thalassoma lunare) on pelagic thesher sharks (Alopias pelagicus) Katarina Grepp Degree project in biology, Master of science (2 years), 2018 Examensarbete i biologi 45 hp till masterexamen, 2018 Biology Education Centre Supervisors: Simon Oliver and Fredrik Sundström 1 Table of contents Abstract ................................................................................................................................................... 3 1. Introduction .................................................................................................................................... 4 2. Method ............................................................................................................................................ 7 2.1 Location ......................................................................................................................................... 7 2.2 Sampling ........................................................................................................................................ 7 2.3 Distinguishing of behaviour and species ....................................................................................... 7 2.4 Analysing ....................................................................................................................................... 8 2.5 Fish survey .................................................................................................................................... -

Marine Biodiversity Survey Final Report
MARINE BIODIVERSITY SURVEY FINAL REPORT A CEPA-JICA PROJECT ON BIODIVERSITY CONSERVATION THROUGH IMPLEMENTATION OF THE PNG POLICY ON PROTECTED AREAS APRIL 2018 Prepared by the Centre for Biodiversity and Natural Products, University of Papua New Guinea Contents EXECUTIVE SUMMARY..................................................................................................................................... 1 CHAPTER 1. MARINE BIODIVERSITY SURVEY OVERVIEW ................................................................................ 5 Introduction ................................................................................................................................................. 5 Sites description .......................................................................................................................................... 6 Geographical Location ............................................................................................................................. 6 Geology .................................................................................................................................................... 7 Marine Environment ............................................................................................................................... 8 Climate..................................................................................................................................................... 8 Vegetation ..............................................................................................................................................