TITLE: Complete Blood Count (CBC) Basics PRESENTER: Sarah Drawz and Michael Linden
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Section 8: Hematology CHAPTER 47: ANEMIA
Section 8: Hematology CHAPTER 47: ANEMIA Q.1. A 56-year-old man presents with symptoms of severe dyspnea on exertion and fatigue. His laboratory values are as follows: Hemoglobin 6.0 g/dL (normal: 12–15 g/dL) Hematocrit 18% (normal: 36%–46%) RBC count 2 million/L (normal: 4–5.2 million/L) Reticulocyte count 3% (normal: 0.5%–1.5%) Which of the following caused this man’s anemia? A. Decreased red cell production B. Increased red cell destruction C. Acute blood loss (hemorrhage) D. There is insufficient information to make a determination Answer: A. This man presents with anemia and an elevated reticulocyte count which seems to suggest a hemolytic process. His reticulocyte count, however, has not been corrected for the degree of anemia he displays. This can be done by calculating his corrected reticulocyte count ([3% × (18%/45%)] = 1.2%), which is less than 2 and thus suggestive of a hypoproliferative process (decreased red cell production). Q.2. A 25-year-old man with pancytopenia undergoes bone marrow aspiration and biopsy, which reveals profound hypocellularity and virtual absence of hematopoietic cells. Cytogenetic analysis of the bone marrow does not reveal any abnormalities. Despite red blood cell and platelet transfusions, his pancytopenia worsens. Histocompatibility testing of his only sister fails to reveal a match. What would be the most appropriate course of therapy? A. Antithymocyte globulin, cyclosporine, and prednisone B. Prednisone alone C. Supportive therapy with chronic blood and platelet transfusions only D. Methotrexate and prednisone E. Bone marrow transplant Answer: A. Although supportive care with transfusions is necessary for treating this patient with aplastic anemia, most cases are not self-limited. -

A Rapid and Quantitative D-Dimer Assay in Whole Blood and Plasma on the Point-Of-Care PATHFAST Analyzer ARTICLE in PRESS
MODEL 6 TR-03106; No of Pages 7 ARTICLE IN PRESS Thrombosis Research (2007) xx, xxx–xxx intl.elsevierhealth.com/journals/thre REGULAR ARTICLE A rapid and quantitative D-Dimer assay in whole blood and plasma on the point-of-care PATHFAST analyzer Teruko Fukuda a,⁎, Hidetoshi Kasai b, Takeo Kusano b, Chisato Shimazu b, Kazuo Kawasugi c, Yukihisa Miyazawa b a Department of Clinical Laboratory Medicine, Teikyo University School of Medical Technology, 2-11-1 Kaga, Itabashi, Tokyo 173-8605, Japan b Department of Central Clinical Laboratory, Teikyo University Hospital, Japan c Department of Internal Medicine, Teikyo University School of Medicine, Japan Received 11 July 2006; received in revised form 7 December 2006; accepted 28 December 2006 KEYWORDS Abstract The objective of this study was to evaluate the accuracy indices of the new D-Dimer; rapid and quantitative PATHFAST D-Dimer assay in patients with clinically suspected Deep-vein thrombosis; deep-vein thrombosis (DVT). Eighty two consecutive patients (34% DVT, 66% non-DVT) Point-of-care testing; with suspected DVT of a lower limb were tested with the D-Dimer assay with a Chemiluminescent PATHFAST analyzer. The diagnostic value of the PATHFAST D-Dimer assay (which is immunoassay based on the principle of a chemiluminescent enzyme immunoassay) for DVT was evaluated with pre-test clinical probability, compression ultrasonography (CUS). Furthermore, each patient underwent contrast venography and computed tomogra- phy, if necessary. The sensitivity and specificity of the D-Dimer assay using 0.570 μg/ mL FEU as a clinical cut-off value was found to be 100% and 63.2%, respectively, for the diagnosis of DVT, with a positive predictive value (PPV) and negative predictive value (NPV) of 66.7% and 100%, respectively. -

We Get Biased on Things That Make Sense
DIALOGUE AND DISCUSSION We Get Biased on Things That Make Sense MERIH T. TESFAZGHI When I was teaching third year medical students, and bench- reaction might be characterized by a marked left shift lecturing clinical laboratory science students, I emphasized which may display a range of immature cells that are also the importance of clinical details and the history of patients seen in CML. Severe left shift especially in the absence of in laboratory test processing, especially in the evaluation of evident infection (or other underlying diseases) may peripheral blood films. During those times, one of my strongly suggest direct bone marrow involvement. In students stated, “but including details of patients might such scenario, clinical details of patients such as absence somehow affect the decision of the reviewer, and may lead to or presence of enlargement of extramedullar organs is Downloaded from a bias.” I responded that we are biased on things that make highly sought. sense. This means that we decide based on the evidence we see from a microscopy evaluation in the light of the clinical Ø The coexistence of multiple conditions in a given detail and history. How do clinical details and history help us patient. One condition may mask the presence of the reach decisions? other condition. For example, the coexistence of megaloblastic anemia with iron deficiency anemia. http://hwmaint.clsjournal.ascls.org/ If specimens are the “in vitro ambassadors” of patients to the Usually, megaloblastic anemia is characterized by laboratory, then properly completed request forms are their increased size of red blood cells (MCV), but when “credentials”. -

Introduction to Hematological Assessment (PDF)
UPDATED 01/2021 Introduction to Hematological Assessment Objectives After completing this lesson, you will be able to: • State the main purposes of a hematological assessment. • Explain the procedures for collecting and processing a blood sample. • Understand the importance of hand washing. • Understand the importance of preventing blood borne pathogen transmission. • Describe and follow the hemoglobin test procedure using the Hemocue Hemoglobin Analyzer. Overview The most common form of nutritional deficiency is “iron deficiency”. It is observed more frequently among children and women of childbearing age (particularly pregnant women). Iron deficiency can result in developmental delays and behavioral disturbance in children, as well as increased risk for a preterm delivery in pregnant women. Iron status can be determined using several different types of laboratory tests. The two tests most commonly used to screen for iron deficiency are hemoglobin (Hgb) concentration and hematocrit (Hct). Proper screening for iron deficiency requires sound laboratory methods and procedures. Often, CPAs will hear the following questions: “Do you have to stick my finger? What does this have to do with my WIC foods anyway? Will it hurt?” For most of us, the thought of having blood taken, even from a finger, is not pleasant. However, evaluating the results of a blood test is a part of screening for nutritional risk. Why Does WIC Require Hematological Assessment? WIC requires that each applicant be screened for risk of a medical condition known as iron deficiency anemia. Anemia is a condition of the blood in which the amount of hemoglobin falls below a level considered desirable for good health. -

Your Blood Cells
Page 1 of 2 Original Date The Johns Hopkins Hospital Patient Information 12/00 Oncology ReviseD/ RevieweD 6/14 Your Blood Cells Where are Blood cells are made in the bone marrow. The bone marrow blood cells is a liquid that looks like blood. It is found in several places of made? the body, such as your hips, chest bone and the middle part of your arm and leg bones. What types of • The three main types of blood cells are the red blood cells, blood cells do the white blood cells and the platelets. I have? • Red blood cells carry oxygen to all parts of the body. The normal hematocrit (or percentage of red blood cells in the blood) is 41-53%. Anemia means low red blood cells. • White blood cells fight infection. The normal white blood cell count is 4.5-11 (K/cu mm). The most important white blood cell to fight infection is the neutrophil. Forty to seventy percent (40-70%) of your white blood cells should be neutrophils. Neutropenia means your neutrophils are low, or less than 40%. • Platelets help your blood to clot and stop bleeding. The normal platelet count is 150-350 (K/cu mm). Thrombocytopenia means low platelets. How do you Your blood cells are measured by a test called the Complete measure my Blood Count (CBC) or Heme 8/Diff. You may want to keep track blood cells? of your blood counts on the back of this sheet. What When your blood counts are low, you may become anemic, get happens infections and bleed or bruise easier. -
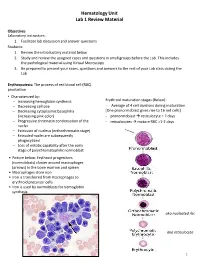
Hematology Unit Lab 1 Review Material
Hematology Unit Lab 1 Review Material Objectives Laboratory instructors: 1. Facilitate lab discussion and answer questions Students: 1. Review the introductory material below 2. Study and review the assigned cases and questions in small groups before the Lab. This includes the pathological material using Virtual Microscopy 3. Be prepared to present your cases, questions and answers to the rest of your Lab class during the Lab Erythropoiesis: The process of red blood cell (RBC) production • Characterized by: − Increasing hemoglobin synthesis Erythroid maturation stages (Below): − Decreasing cell size - Average of 4 cell divisions during maturation − Decreasing cytoplasmic basophilia [One pronormoblast gives rise to 16 red cells] (increasing pink color) - pronormoblast → reticulocyte = 7 days − Progressive chromatin condensation of the - reticulocytes → mature RBC =1-2 days nuclei − Extrusion of nucleus (orthochromatic stage) − Extruded nuclei are subsequently phagocytized − Loss of mitotic capability after the early stage of polychromatophilic normoblast • Picture below: Erythroid progenitors (normoblasts) cluster around macrophages (arrows) in the bone marrow and spleen • Macrophages store iron • Iron is transferred from macrophages to erythroid precursor cells • Iron is used by normoblasts for hemoglobin synthesis aka nucleated rbc aka reticulocyte 1 Mature Red Blood Cell 7-8 microns; round / ovoid biconcave disc with orange-red cytoplasm, no RNA, no nucleus; survives ~120 days in circulation Classification of Anemia by Morphology 1. -

Copyrighted Material
CHAPTER 1 The Blood Film and Count Blood Blood is a life‐sustaining fluid that circulates through the heart and blood vessels. It carries oxygen and nutrients to the tissues and waste products to the lungs, liver and kidneys, where they can be removed from the body. Usually when blood is removed from the body it forms a solid blood clot. However, if clotting is prevented by mixing with an anticoagulant, the blood separates, under the influence of gravity, into three layers (Fig. 1.1). The bottom layer is deep red in colour and is composed of red cells. The top layer is clear and pale yellow. It is called plasma and is composed of various salts and proteins dissolved in water. In between is a narrow layer called the buffy coat because of its buff or yellowish white colour. The buffy coat is composed mainly of cells of a variety of types, collectively known as white cells. In addition there are small cellular fragments, called platelets, which have a role in blood clotting. The bloodCOPYRIGHTED film MATERIAL Although we can judge the proportions of red cells and white cells in a tube of sedimented blood, we get far more information if the blood is carefully mixed and a thin layer is spread on a glass A Beginner’s Guide to Blood Cells, Third Edition. Barbara J. Bain. © 2017 John Wiley & Sons Ltd. Published 2017 by John Wiley & Sons Ltd. 1 0003049575.indd 1 2/24/2017 9:50:31 AM 2 A Beginner’s Guide to Blood Cells Plasma Buffy coat Red cells Fig. -

Blood Film Preparation and Staining Procedures
INTERPRETATION OF THE PERIPHERAL 00. ם BLOOD FILM 0272–2712/02 $15.00 BLOOD FILM PREPARATION AND STAINING PROCEDURES Berend Houwen, MD, PhD The blood film is one of the world’s most widely and frequently used tests and has undergone remarkably few changes since its introduction as a clinical diagnostic tool in the late 1800s. The origins of stained blood film microscopy are somewhat obscure, and a clear reference for the ‘‘first person or persons’’ to describe its use as a clinical laboratory procedure cannot be established with certainty. Antonie van Leeuwenhoek, in the seventeenth century, was the first to describe blood cells, using whole blood preparations and not blood films for his observations on blood corpuscles. In fact, he would not have been able to use blood film–based microscopy because it requires considerably more sophisti- cated optics than were available in his day. A second technologic innovation enabling blood film microscopy was the introduction of aniline dyes in the second half of the nineteenth century. This made it possible to study individual blood cells by light microscopy after a small amount of blood had been placed and smeared onto glass slides, dried, fixed, and then stained. Paul Ehrlich introduced eosin as the first of these dyes for staining blood films in 1856, followed by hematoxylin in 1865, and later by the metachromatic Romanowsky dyes. A few refinements have since been made, consisting mainly of improve- ments in dye quality, staining procedures, automation of slide preparation, and staining, but the basic elements of blood film preparation and analysis have not changed for over a century. -

Blood Donor Information
Blood Donor Information Thank you for coming to donate blood. We are Did you know that some causes sorry that we cannot accept you as a donor today of anemia are invisible? because your hematocrit level was too low. You may not know you have bleeding from We care about your health and want to help you your digestive tract. This can be a result of: understand what having a low hematocrit level means.You are not alone, having a low hematocrit • Stomach ulcers level is the most frequent cause for not being able • Growths in intestines (polyps) to donate blood. You may return to donate when • Colon cancer your hematocrit level has increased and you can • Certain medications help immediately by asking your friends, family • Other diseases of the digestive tract and/or coworkers to donate. There are several Anemia is often the first symptom of these simple ways to improve your hematocrit level. conditions so it should be taken seriously. If you feel You may be eligible to donate another time. you don’t fit in any of the previous categories, What is Hematocrit? or just want to rule out these possibilities, make an appointment with your physician without delay. Blood is made up of red blood cells, white blood cells, platelets, and plasma. Hematocrit is the percentage of blood You should see your doctor if your volume that is red blood cells. Red blood cells contain hematocrit is low and you: hemoglobin which carries oxygen to all the cells in the body. • donate less than three times per year, Why do we test potential blood • are a non-menstruating woman of any donors for hematocrit levels? age with a hematocrit below 36%, Hematocrit is measured prior to each donation as part of • a man with a hematocrit less than 38%, and donor screening. -
Complete Blood Count in Primary Care
Complete Blood Count in Primary Care bpac nz better medicine Editorial Team bpacnz Tony Fraser 10 George Street Professor Murray Tilyard PO Box 6032, Dunedin Clinical Advisory Group phone 03 477 5418 Dr Dave Colquhoun Michele Cray free fax 0800 bpac nz Dr Rosemary Ikram www.bpac.org.nz Dr Peter Jensen Dr Cam Kyle Dr Chris Leathart Dr Lynn McBain Associate Professor Jim Reid Dr David Reith Professor Murray Tilyard Programme Development Team Noni Allison Rachael Clarke Rebecca Didham Terry Ehau Peter Ellison Dr Malcolm Kendall-Smith Dr Anne Marie Tangney Dr Trevor Walker Dr Sharyn Willis Dave Woods Report Development Team Justine Broadley Todd Gillies Lana Johnson Web Gordon Smith Design Michael Crawford Management and Administration Kaye Baldwin Tony Fraser Kyla Letman Professor Murray Tilyard Distribution Zane Lindon Lyn Thomlinson Colleen Witchall All information is intended for use by competent health care professionals and should be utilised in conjunction with © May 2008 pertinent clinical data. Contents Key points/purpose 2 Introduction 2 Background ▪ Haematopoiesis - Cell development 3 ▪ Limitations of reference ranges for the CBC 4 ▪ Borderline abnormal results must be interpreted in clinical context 4 ▪ History and clinical examination 4 White Cells ▪ Neutrophils 5 ▪ Lymphocytes 9 ▪ Monocytes 11 ▪ Basophils 12 ▪ Eosinophils 12 ▪ Platelets 13 Haemoglobin and red cell indices ▪ Low haemoglobin 15 ▪ Microcytic anaemia 15 ▪ Normocytic anaemia 16 ▪ Macrocytic anaemia 17 ▪ High haemoglobin 17 ▪ Other red cell indices 18 Summary Table 19 Glossary 20 This resource is a consensus document, developed with haematology and general practice input. We would like to thank: Dr Liam Fernyhough, Haematologist, Canterbury Health Laboratories Dr Chris Leathart, GP, Christchurch Dr Edward Theakston, Haematologist, Diagnostic Medlab Ltd We would like to acknowledge their advice, expertise and valuable feedback on this document. -

116588NCJRS.Pdf
If you have issues viewing or accessing this file contact us at NCJRS.gov. " "'- u.s. Department of Justice Federal Bureau of Investigation PROCEEDINGS OF THE INTERNATIONAL SYMPOSIUM ON FORENSIC IMMUNOLOGY FBI ACADEMY QUANTICO, VIRGINIA JUNE 23-26, 1986 Proceedings of the International Symposium on Forensic Immunology co 00 L() \0 u, r-l O§ r-l Ql C. If) 05~ Ql 0- 0 ~ o~ Ql E C/) Ql >. c:0 If) C/) ~ * ....,a: () a: z Ql '*02 Ql iii :5 ...... ....,::> a Ql {ij "...:0- Ql c: !!lc: °E ::>;: B 00 {ij c: 0 ~I::> z~ "e CD ~~ :5 .... ~o- S 1::C: Lt .~ Host Laboratory Division Federal Bureau of Investigation June 23-26, 1986 Forensic Science Research and Training Center FBI Academy Quantico, Virginia NOTICE This publication was prepared by the U. S. Government. Neither the U. S. Government nor the U. S. Department of Justice nor any of their employees makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness or usefulness of any information, apparatus, product or process disclosed, or represents that in use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, mark, manufacturer or otherwise does not necessarily constitute or imply its endorsement, recommendation or favoring by the U. S. Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the U. S. Government or any agency thereof. Published by: The Laboratory Division Roger T. Castonguay Assistant Director in Charge Federal Bureau of Investigation U. -

Understanding Blood Tests
PATIENT EDUCATION patienteducation.osumc.edu Understanding Blood Tests Your heart pumps the blood in your body through a system of blood vessels. Blood delivers oxygen and nutrients to all parts of the body. It also carries away waste products. Blood is made up of red blood cells, white blood cells, platelets and a clear fluid called plasma. There are also other parts in the blood such as electrolytes, proteins, fats, sugar, and hormones. Your doctor can check for certain diseases and conditions by doing blood tests. A small sample of blood is taken and sent to the lab. The test results help your doctor: • Check your general health • See how well your body organs are working • Find health problems, diseases and disorders • Watch the body’s response to medicines and treatments Common blood tests include a Complete Blood Count (CBC) with differential, and a Comprehensive Metabolic Panel (CMP). Note: Some health problems may require ongoing monitoring or repeat testing. It is important to know that normal range values can vary from laboratory to laboratory. Talk with your doctor if you have questions about your test results. Complete Blood Count (CBC) A CBC shows the number of white and red blood cells, hematocrit, hemoglobin and platelets in your blood. This test helps your doctor look for signs of anemia, infection, bleeding problems or certain diseases and disorders. This handout is for informational purposes only. Talk with your doctor or health care team if you have any questions about your care. © January 9, 2018. The Ohio State University Comprehensive Cancer Center – Arthur G.