Sol–Gel Process and Complex Fluids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Research and Innovation Capabilities : Insights on India's Plunge Into Nanotechnology
Research and Innovation Capabilities : Insights on India’s plunge into Nanotechnology Anand, Manish The Energy and Resources Institute, India 1. Introduction Nanotechnology is expected to be one of the main drivers of future industries‟ development and economic growth (see, for instance, Huang et al., 2004; Hullmann, 2007; Kostoff et al., 2007; Meyer et al., 2008; Salerno et al., 2008 ; Roco et al., 2010). Nanotechnology‟s significant potential for technological change and for current and future world markets becomes apparent when looking at its market size and its growth potential. Estimates by market research and industry analysis show that the market for the nanotechnology was 11.67 billion US$ in 2009 and is expected to grow at a CAGR of around 17.5% during 2016-2022 (BCC, 2010; RNCOS, 2015). The promise of the socio-economic benefits that could be derived from nanotechnology has resulted in both developed and developing country investment and R&D engagement with nanotechnology, as the UNESCO Science Report: Towards 2030 explains. Indeed, the USA formally marked the start of an international technology race in nanotechnology, with the launching of its „National Nanotechnology Initiative‟ in January 2000, consecrating the phenomenal amount of US$270 million to this promising field. The rest of the world has followed suite with minimal delay – presumably to avoid the technological lag. The EU Framework Program for Research and Technological Development in Europe made it one of its themes in the 5th Framework Programme (1998–2002), with an overall budget of €14.96 billion. Developing and transition economies of Brazil, Russia, India, China, and South Africa (BRICS) have also given nanotechnology a high priority, and all countries have defined a national plan for its development. -
Outline of Selected Projects Comments by Program Committee
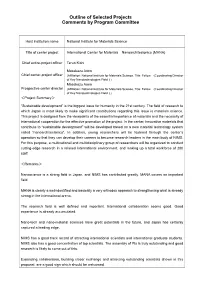
Outline of Selected Projects Comments by Program Committee Host institution name National Institute for Materials Science Title of center project International Center for Materials Nanoarchitectonics (MANA) Chief entire-project officer Teruo Kishi Masakazu Aono Chief center-project officer (Affiliation: National Institute for Materials Science, Title: Fellow (Coordinating Director of Key Nanotechnologies Field ) ) Masakazu Aono Prospective center director (Affiliation: National Institute for Materials Science, Title: Fellow (Coordinating Director of Key Nanotechnologies Field ) ) <Project Summary> “Sustainable development” is the biggest issue for humanity in the 21st century. The field of research to which Japan is most likely to make significant contributions regarding this issue is materials science. This project is designed from the viewpoints of the essential importance of materials and the necessity of international cooperation for the effective promotion of the project. In the center, innovative materials that contribute to “sustainable development” will be developed based on a new material technology system called “nanoarchitectonics”. In addition, young researchers will be fostered through the center’s operation so that they can develop their careers to become research leaders in the main body of NIMS. For this purpose, a multinational and multidisciplinary group of researchers will be organized to conduct cutting-edge research in a relaxed international environment, and making up a total workforce of 200 staff. <Remarks> Nanoscience is a strong field in Japan, and NIMS has contributed greatly. MANA covers an important field. MANA is clearly a well-identified and basically a very orthodox approach to strengthening what is already strong in the international arena. The research field is well defined and important. -

Sol-Gel Engineering Reviewed C
Sol-gel engineering to tune structural colours Marco Faustini To cite this version: Marco Faustini. Sol-gel engineering to tune structural colours. Journal of Sol-Gel Science and Tech- nology, Springer Verlag, 2020, 10.1007/s10971-020-05319-7. hal-02895489 HAL Id: hal-02895489 https://hal.sorbonne-universite.fr/hal-02895489 Submitted on 9 Jul 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Sol-gel engineering to tune structural colours Marco Faustini1* 1Sorbonne Université, CNRS, Collège de France UMR 7574 Chimie de la Matière Condensée de Paris, F-75005 Paris, France [email protected] Abstract For several decades, the sol-gel process allows the fabrication of functional materials through the strong coupling between materials chemistry and advanced processing. This branch of material’s science is generally associated to the formation of oxide and hybrid materials obtained through a transition between a sol and a gel. Engineering the sol-gel process to extend same principles to other classes of materials is an emerging and promising research line. These aspects will be illustrated in this article by reviewing the successful case of optical materials with tunable structural colors. -

Osaka University Knowledge Archive : OUKA
Title MEMOIRS of the Institute of Scientific and Industrial Research, Osaka University Volume 68 Author(s) MEMOIRS of the Institute of Scientific and Citation Industrial Research, Osaka University. 68 P.1- P.253 Issue Date 2011 Text Version publisher URL http://hdl.handle.net/11094/77448 DOI rights Note Osaka University Knowledge Archive : OUKA https://ir.library.osaka-u.ac.jp/ Osaka University Contents Foreword ················································································ 1 Outline of ISIR 1. Research Activities ······································································· 2 2. Education ·················································································· 17 3. International Exchange ·································································· 18 4. Concluding Remarks ····································································· 20 Activities of Divisions Division of Information and Quantum Sciences ········································ 25 Division of Advanced Materials and Beam Science ···································· 42 Division of Biological and Molecular Sciences ········································· 57 Specially Appointed Laboratory ··························································· 72 Division of Next Industry Generation ···················································· 74 Division of Special Projects ······························································· 76 Department of Disease Glycomics ························································ -

Professor Justin J. Cooper-White
PROFESSOR JUSTIN J. COOPER-WHITE Professor Cooper-White currently holds positions of Associate Dean (Research) of the Faculty of Engineering, Architecture and IT (University of Queensland), Professor within the School of Chemical Engineering (University of Queensland), Group Leader within the Australian Institute for Bioengineering and Nanotechnology (University of Queensland) and Director of the Australian National Fabrication Facility (Queensland Node). Current research interests include the development of novel biomaterials and engineered surfaces for tissue engineered cartilage, bone, cardiac muscle and vascular systems, microbioreactors for stem cell expansion and differentiation, microfluidic devices for biofluid property measurement and early disease detection and the manufacture of microparticle delivery systems. He has authored or co-authored over 250 research publications and presentations and is often asked to present plenary, keynote and invited lectures at national and international conferences. Since gaining his Ph.D. in 2000, his career citations total to 1516, and he has an h-index of 22 (scopus, 28/11/11). He is the past President of both The Australasian Society for Biomaterials and Tissue Engineering and The Australian Society of Rheology, a consultant for a number of national and international companies, associate editor of the Korean-Australian Rheology Journal, on the editorial boards of Soft Materials, Biomicrofluidics and Rheologica Acta, and a reviewer for major international journals in his fields of expertise. He holds 7 Int. patents in the areas of formulation design for agriproducts, microbioreactors, particle synthesis using microfluidic devices and tissue engineering scaffolds. Relevant employment history • Professor. The University of Queensland, 2007-present • Associate Professor, The University of Queensland, 2004-2006 • Senior Lecturer, The University of Melbourne, 2003-2004. -

NEWSLETTER Advanced Materials Research Institute
NEWSLETTER Advanced Materials Research Institute Volume 15, Issue 2 http://www.uno.edu/amri June 2017 THE DIRECTOR'S CORNER New Start-Up Company Works Side by Side with AMRI Greetings! This quarter has brought an exciting “advance” to AMRI. We have Through a University of Louisiana system welcomed Advano, a renewable energy approved collaborative agreement, the startup start-up. This is especially eventful because company, Advano, is now renting space in it represents a new paradigm in AMRI to further their efforts to establish collaborations for AMRI and UNO. Advano themselves as a new leader in the development is working as an independent company of the next generation of anode components for renting lab space in AMRI, while at the rechargeable batteries. Success in these same time, they are able to readily access materials could increase the energy storage AMRI equipment and collaborate with AMRI and lifetimes of batteries by several fold. So far researchers. AMRI benefits from equipment Advano has over $500 K in committed financial use fees and more importantly from the fast support from venture capitalists. developing collaborations with Advano. (http://www.advanotech.com/) Plans are already in the works for AMRI researchers to pursue funding opportunities with Advano through the Louisiana Board of Regents Industrial Ties Program. Another success this quarter has been the return of both our undergraduate and high Other companies rent space on campus but school summer research programs. These this collaborative agreement with Advano programs have run continuously for 14 and represents a new paradigm in that these 15 years, respectively. -

Download Data Or Protocols from the NFFA Data Repository, He/She Will Be Asked to Formally Accept the Rules and Conditions Set by the NFFA DR
nanoscience foundries and fi ne analysis A EUROPEAN OPEN-ACCESS DISTRIBUTED RESEARCH INFRASTRUCTURE FOR NANOSCIENCE AND ADVANCED ANALYSIS 7th Framework Programme – Capacities – Design Studies – Project No . FP7 – 212348 Cristina Africh1, Heinz Amenitsch5, Graham Arthur2, Fernando Cacho-Nerin5, Regina Ciancio1, Dan Cojoc1, Stefano Cozzini1, Zheng Cui2, Christian David4, Stefano Fabris1, Roberta Ferranti1, Luis Fonseca3, Jordi Fraxedas3, Jens Gobrecht4, Roberto Gotter1, Justin Greenhalgh2, Ejaz Huq2, Karin Jungnikl5, Peter Laggner5, Emilio Lora-Tamayo3, Benedetta Marmiroli5, Daniela Orani1, Giancarlo Panaccione1, Michael Rappolt5, Giorgio Rossi1, Barbara Sartori5, Massimo Tormen1 1CNR-IOM Consiglio Nazionale delle Ricerche Istituto Officina dei Materiali 2STFC Science and Technology Facilities Council 3CSIC-CNM Consejo Superior de Investigaciones Cientificas Centro Nacional de Microelectronica on behalf of BNC-b (Barcelona Nanocluster-Bellaterra) 4PSI Paul Scherrer Institute Laboratory for Micro- and Nanotechnology 5OEAW Austrian Academy of Sciences Institute of Biophysics and Nanosystems Research FOREWORD Nanoscience and nanotechnology are broad domains of research and innovation that represent a major fi eld of activity at global level. This originates from the outstanding demonstrations obtained in the last two decades of the possibility to control the organization of matter at the scale of few atoms and molecules, to address their properties and to obtain functional systems with potential exploitation in the fi elds of health, environment, energy and communication. Research in nanoscience requires direct sensitivity to the atomic scale and to the fundamental time scale of the processes ruling the assembly of matter and the interactions with light, electric current, and molecules at all levels of complexity, from the gases of the atmosphere to proteins and DNA. -

Surfactants, Ionic Liquids and Ionosilicas: Functional Ionic Systems for Supramolecular Chemistry and Elaboration of Materials B
Surfactants, Ionic liquids and Ionosilicas : functional ionic systems for supramolecular chemistry and elaboration of materials by design (ion exchange and vectorization) Roza Bouchal To cite this version: Roza Bouchal. Surfactants, Ionic liquids and Ionosilicas : functional ionic systems for supramolecular chemistry and elaboration of materials by design (ion exchange and vectorization). Material chemistry. Université Montpellier, 2016. English. NNT : 2016MONTT203. tel-01758252 HAL Id: tel-01758252 https://tel.archives-ouvertes.fr/tel-01758252 Submitted on 4 Apr 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Délivré par l’Université de Montpellier Préparée au sein de l’école doctorale Sciences Chimiques Balard Et de l’unité de recherche Chimie Moléculaire et Organisation du Solide (ICGM) Spécialité : Chimie et Physicochimie des Matériaux Présentée par Roza Bouchal Surfactants, Ionic liquids and Ionosilicas: Functional ionic systems for supramolecular chemistry and elaboration of materials by design (ion exchange and vectorization) Soutenue le 19 Octobre 2016 devant le jury composé de Mme Marie-Helène DELVILLE, DR, Université de Bordeaux Rapporteur M. Giang VO THANH, Professeur, Université Paris Paris-Sud 11 Rapporteur M. Jean-Olivier DURAND, DR, Université de Montpellier Président du jury M. -

Abstracts 2010 Euvl
2010 International Workshop on EUV Lithography June 21-25, 2010 Makena Beach Golf Resort ▪ Maui, Hawaii Workshop Agenda 2010 International Workshop on EUV Lithography Contents Welcome ______________________________________________________ 3 Workshop Agenda _______________________________________________ 5 Abstracts by Technical Area ________________________________________ 20 Organized by: www.euvlitho.com 1 2010 International Workshop on EUV Lithography GOLD LEVEL SPONSORS XEI SCIENTIFIC, INC. PLATINUM LEVEL SPONSORS www.euvlitho.com 2 2010 International Workshop on EUV Lithography Welcome Dear Colleagues; I will like to welcome you to the 2010 International Workshop on EUV Lithography in Maui, Hawaii. In this leading workshop, focused entirely on EUVL R&D, researchers from around the world will present the results of their R&D. As we all work to address the remaining technical challenges of EUVL to allow its insertion in high volume computer chip manufacturing, we look forward to a productive interaction among colleagues to brainstorm technical solutions. This workshop has been made possible by the support of workshop sponsors, steering committee members, workshop support staff, session chairs and presenters. I would like to thank them for their contributions and making this workshop a success. I look forward to your participation in the workshop. Best Regards Vivek Bakshi Organizing Chair, 2010 International Workshop on EUVL www.euvlitho.com 3 2010 International Workshop on EUV Lithography EUVL Workshop Steering Committee Executive -

Download (228Kb)
Workshop Summary____ Workshop Report: Synergies in NanoScale Manufacturing & Research held at Cornell University, Ithaca, NY, January 28 and 29, 2010 Sponsored by NNIN, NNN2, and CNF1 Summary Prepared by: Alan Bleier1, Don Tennant1, and Sandip Tiwari1 1 Cornell University, Ithaca , NY 2 University of Massachusetts, Amherst, MA I. Executive Summary CNF hosted a two day workshop, “Synergies in NanoScale Manufacturing and Research,” held on the Cornell University campus January 28-29, 2010. This was a by-invitation-only working group intended to generate active discussion in the issues related to bringing emergent tools, processes, and materials into commercialization. The speakers, moderators, and attendees were selected from across the country and from industry, academia, and government labs to bring a broad range of expertise and experience to the group discussions. The workshop was sponsored jointly by the NSF’s National Nanomanufacturing Network (centered at U Mass Amherst), and the National Nanotechnology Infrastructure Network. The program effort was led by Don Tennant, Sandip Tiwari , and Lynn Rathbun from Cornell University and Mark Tuominen and Jeff Morse from U. Mass. Speaker topics ranged from roll to roll production of flexible electronics, mass methods of producing bit patterned magnetic media, ways to manufacture in silicon with atomic precision to groundbreaking methods of making measurements of structural properties in complex materials. We also heard reports on several new means of highly specialized drug delivery, the possibility of mass production of carbon substrates for electronics, a new class of photochemicals compatible with organic materials, and more. Each day’s presentations were followed by group and breakout discussions centered on questions and topics stimulated by the various speakers. -

2011 Euvl Workshop Sponsors
2011 International Workshop on EUV Lithography June 13-17, 2011 Makena Beach Golf Resort ▪ Maui, Hawaii Workshop Abstracts 2011 International Workshop on EUV Lithography Contents Welcome ______________________________________________________ 3 Workshop Agenda _______________________________________________ 5 Abstracts by Technical Area ________________________________________ 20 Organized by: www.euvlitho.com 1 2011 International Workshop on EUV Lithography 2011 EUVL WORKSHOP SPONSORS Gold Level Sponsors XEI SCIENTIFIC, INC. Platinum Level Sponsors www.euvlitho.com 2 2011 International Workshop on EUV Lithography Welcome Dear Colleagues; I would like to welcome you to the 2011 International Workshop on EUV Lithography in Maui, Hawaii. In this leading workshop, focused entirely on EUVL R&D, researchers from around the world will present the results of their R&D. As we all work to address the remaining technical challenges of EUVL to allow its insertion in high volume computer chip manufacturing, we look forward to a productive interaction among colleagues to brainstorm technical solutions. This workshop has been made possible by the support of workshop sponsors, steering committee members, workshop support staff, session chairs and presenters. I would like to thank them for their contributions and making this workshop a success. I look forward to your participation. Best Regards Vivek Bakshi Organizing Chair, 2011 International Workshop on EUVL www.euvlitho.com 3 2011 International Workshop on EUV Lithography EUVL Workshop Steering Committee -

4. European Research Infrastructures (Including E-Infrastructures) Revised
EN HORIZON 2020 WORK PROGRAMME 2014 – 2015 4. European research infrastructures (including e-Infrastructures) Revised This Work Programme was adopted on 10 December 2013. The parts that relate to 2015 (topics, dates, budget) have, with this revised version, been updated. The changes relating to this revised part are explained on the Participant Portal. (European Commission Decision C (2015)2453 of 17 April 2015) HORIZON 2020 – WORK PROGRAMME 2014-2015 European research infrastructures (including e-Infrastructures) Table of Contents Introduction .............................................................................................................................. 4 Call - Developing new world-class research infrastructures ................................................ 6 INFRADEV-1-2014: Design Studies ................................................................................................. 6 INFRADEV-2-2015: Preparatory Phase of ESFRI projects .............................................................. 7 INFRADEV-3-2015: Individual implementation and operation of ESFRI projects .......................... 9 INFRADEV-4-2014/2015: Implementation and operation of cross-cutting services and solutions for clusters of ESFRI and other relevant research infrastructure initiatives .................................... 10 CONDITIONS FOR THIS CALL ...................................................................................................... 13 Call - Integrating and opening research infrastructures of European interest