3% Sorbitol Urologic Irrigating Solutionin UROMATIC Plastic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

224.50-585 Plastic Resin Code Labeling. (1) As Used in This Section: (A) "Rigid Plastic Container" Means Any Formed
224.50-585 Plastic resin code labeling. (1) As used in this section: (a) "Rigid plastic container" means any formed or molded article comprised predominantly of plastic resin and having a relatively inflexible finite shape or form intended primarily as a single service container with a capacity of eight (8) ounces or more and less than five (5) gallons; (b) "Rigid plastic bottle" means any rigid plastic container with a neck that is smaller than the container body with a capacity of sixteen (16) ounces or more and less than five (5) gallons; and (c) "Label" means a code label described in this section molded into the bottom of the plastic product. (2) All rigid plastic bottles and rigid plastic containers sold in Kentucky on and after January 1, 1992, shall be labeled with a code which indicates the resin used to produce the rigid plastic bottle or rigid plastic container. The code shall consist of a number placed inside a triangle and letters placed below the triangle. The triangle shall be equilateral, formed by three (3) arrows with the apex of each point of the triangle at the midpoint of each arrow, rounded with a short radius. The pointer or arrowhead of each arrow shall be at the midpoint of each side of the triangle with a short gap separating the pointer from the base of the adjacent arrow. The triangle, formed by the three (3) arrows curved at their midpoints, shall depict a clockwise path around the code number. The numbers and letters used shall be as follows: 1 = PETE (polyethylene terephthalate) 2 = HDPE (high density polyethylene) 3 = V (vinyl) 4 = LDPE (low density polyethylene) 5 = PP (polypropylene) 6 = PS (polystyrene) 7 = OTHER (represents all other resins, including layered plastics of a combination of materials). -

Plastic Bottles Today Innovating to Reach Today’S Consumer
PLASTICS MARKET WATCH PLASTIC BOTTLES TODAY INNOVATING TO REACH TODAY’S CONSUMER BETTER INDUSTRY. BETTER WORLD. February 2017 The Plastics Industry Association (PLASTICS) sends special thanks to the Brand Owners, Processors and Equipment Councils, and Rigid Plastics Packaging Group (RPPG) for their guidance and input on this Bottling Plastics Market Watch Report. Materials were compiled, written and edited by William (Bill) Mashek, with editorial assistance from Kim Holmes, George Southworth, Kendra Martin and Ashley Stoney at PLASTICS. Copyright Plastics Industry Association. 02 Plastics Market Watch—Watching: Bottling Plastics Market Watch Plastic Bottles Today Innovating to Reach Today’s Consumer A series examining the business of plastics, including demographics, economics, policy developments and technological trends in specific plastics end markets. Contents 05 Forward 08 Introduction: Plastic Bottles Today— Innovating to Reach Today’s Consumer 11 Role of Plastics in Bottling 16 Growth of Plastics Bottling 20 Innovation 25 Plastic Bottle Economics 29 Bottling Equipment & Machinery 31 Recycling Progress 37 Conclusion 42 Plastic Bottle Glossary 49 Sources 52 Plastics Market Watch Snapshot Plastics Market Watch—Watching: Bottling 03 04 Plastics Market Watch—Watching: Bottling Forward The Plastics Industry Association’s (PLASTICS’) Plastics Market Watch reports provide forward-looking data and insights on key plastics industry end markets. The series examines the business of plastics, including demographics, economics, policy developments and technological improvements for markets including automotive and transportation, healthcare and medical devices, packaging, building and construction, automotive recycling, bioplastics and consumer electronics. Given the role that plastics play in today’s modern society, Plastics Market Watch reports offer a holistic picture of our technology—from beginning-of-life resins and polymers to end-of-life management and recycling efforts. -

Why Can't All Plastics Be Recycled?
Why can’t all plastics be recycled? Plastic has become prevalent because it is inexpensive and it can be engineered with a wide range of properties. Plastics are strong but lightweight, somewhat resistant to being degraded by chemicals, sunlight, and bacteria, and are thermally and electrically insulating. What does the symbol mean? A chasing arrows symbol, or resin code, does not mean that a plastic container is recyclable. Most plastic containers are marked with the chasing arrows symbol ‐ number one through seven in the center. The number inside the arrows signifies the main chemical compound used to make that plastic container. Unfortunately, the symbol does not mean that plastic container can be recycled. There are seven resin codes used inside the chasing arrow symbols: 1. PETE Polyethylene Terephthalate is in pop and water bottles. Please recycle. 2. HDPE High Density Polyethylene is opaque and usually in bottles that store laundry detergent and milk. These are usually recyclable. 3. V Polyvinyl Chloride (PVC) is found in plastic pipes, shrink wrap. 4. LDPE Low Density Polyethylene is in produce bags, plastic wrap, and plastic bags. 5. PP Polypropylene is used for yogurt tubs, ketchup bottles. 6. PS Polystyrene is found in Styrofoam, used for egg crates. 7. Other This category covers a vast mixture of resins and includes food containers (clam shells), polycarbonate used in sport bottles, and bio‐based plastic used in compostable food containers. Why don’t we recycle all these plastics? Most cities collect #1 and #2 types of plastic, or the plastic bottles made from PETE/PET and HDPE resin. -
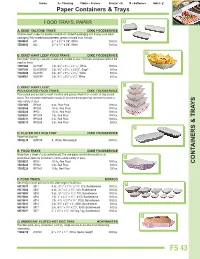
CONTAINERS & TRA YS Paper Containers & Trays
Paper Containers & Trays FOOD TRAYS, PAPER A A. DIXIE® VELTONE TRAYS DIXIE FOODSERVICE Shallow depth makes for excellent individual sandwich packaging and display case meat packaging. Poly-coated board prevents grease or liquid soak through. 1 1 15000641 641 3 /4'' x 5 /4'' x 7/8'', White 1000/cs. 1 1 15000642 642 3 /2'' x 7 /2'' x 5/8'', White 1000/cs. B B. DIXIE® KANT LEEK® FOOD TRAYS DIXIE FOODSERVICE Kant Leek® food trays are poly-coated and durable to resist moisture and grease without the need for liners. 1 11 19 15001666 KL100W 1 lb., 6 /4'' x 4 /16'' x 1 /32'', White 1000/cs. 3 13 ™ 15001595 KL300SAGE 3 lb., 8 /8'' x 5 /16'' x 23/32'', Sage 500/cs. 3 13 3 15004020 KL300W 3 lb., 8 /8'' x 5 /16'' x 2 /32'', White 500/cs. 5 1 9 15000051 KL500W 5 lb., 9 /16'' x 6 /8'' x 2 /32'', White 500/cs. C C. DIXIE® KANT LEEK® POLY-COATED FOOD TRAYS DIXIE FOODSERVICE Poly-coated and durable to resist moisture and grease. Made from a sheet of clay-coated board. The one-piece construction results in an economical open-top container to serve a wide variety of uses. 15001605 RP408 6 oz., Red Plaid 1000/cs. 15000456 RP258 1/4 lb., Red Plaid 1000/cs. 15005220 RP50 1/2 lb., Red Plaid 1000/cs. 15005204 RP1008 1 lb., Red Plaid 1000/cs. 15005211 RP2008 2 lb., Red Plaid 1000/cs. 15005212 RP3008 3 lb., Red Plaid 500/cs. -

Reusable Plastic Container Fact Sheet
Reusable Plastic Container Fact Sheet Reusable Plastic Containers The use of reusable plastic containers(RPC’s) by fruit and vegetable growers has recently become more popular as the push for sustainability increases. As more producers become concerned with decreasing the amount of waste created throughout the supply chain, sustainable options become progressively more relevant. The movement away from corrugated cardboard as the primary containment through transportation and distribution has become a viable RPC Companies option as growers look to RPC There are several different companies companies for a multi-purpose, more that produce and distribute quality sustainable option. RPCs. (Companies not limited to list.) Sustainable Transport Packaging RPCs can be used more successfully Their headquarters are located in St. in different application than Petersburg, FL. with various stocking corrugated cardboard throughout the locations around the U.S. They are supply chain. Growers are able to use a reseller, distributor, and custom RPCs from harvesting the product, manufacturer of various RPCs for a to transportation, to distribution number of applications. and sales as the display making Rehrig Pacific Company their operation more efficient. With their U.S. corporate headquarters This efficiency paired with the Corrugated Cardboard in Los Angeles, CA Rehrig has been sustainability of a product that lasts • Environmental Protection serving the Agriculture industry with for multiple uses, is the very option Agency (EPA) Estimates 30.1 RPCs since the 1980’s. Their products producers are searching for. Million tons of corrugated are designed to help producers safely cardboard are generated each and effectively transport products to RPCs have been around for more year(1) desired markets. -

A COMPARISON of GELATIN and TRADITIONAL PLASTICS Grant Proposal
A COMPARISON OF GELATIN AND TRADITIONAL PLASTICS Grant Proposal Team H: Allison Brookhart, Bao Le, Amelia Navarre, Kelly Simpson, Ame Tsamasse PROJECT SUMMARY To what degree would gelatin plastics be a viable option compared to conventional plastics in the longevity of the plastic function, biodegradability, cleanliness, and in economic feasibility? If gelatin plastics last longer, are more biodegradable, are healthier for humans, and are inexpensive, then they would be a feasible alternative to conventional plastics. Plastic waste and microplastics are a major problem that could have detrimental effects on marine ecosystems, terrestrial animal species, human health, and more. BACKGROUND In Case Study 1, we found there was not a lot of research on gelatin plastics with a hydrophobic coating. We wanted to create gelatin plastics that can do well in moist environments, so as to be suited for the foodservice industry. In the first case study, we created three different coatings for the plastics we made. The coating with beeswax and lignin mixed acetone fared the best out of the three: it was the most hydrophobic and adhered to the plastic the longest. In this case study, we want to continue this research. The questions we are now posting are the unknowns that arose from the previous experiment. This includes asking if there is a way to make a better film that holds for a longer time period and testing the efficiency of the biodegradability of the plastics. Gelatin plastic has long been explored as a safer and more biodegradable product for food packaging. It is a more environmentally-friendly alternative to the plastic used today and could serve as a possible solution to the global plastic epidemic (Hanani et al., 2014). -

Download Catalog
DELIVERING VALUE BEYOND THE BOX BUCKHORN REUSABLE PACKAGING SOLUTIONS Reusable Packaging Solutions Product Catalog PRODUCT CATALOG Table of Contents Buckhorn Inc. Why Buckhorn .......................................................................................... 3-6 400 TechneCenter Drive, Suite 215 Bulk Boxes ..................................................................................................... 7 Milford, OH 45150 Bulk Boxes Overview ....................................................................................... 8-9 Toll Free: (800) 543-4454 General Purpose Boxes ................................................................................ 10-11 Tel: (513) 831-4402 Fax: (513) 831-5474 Standard-Duty Boxes ....................................................................................12-13 Extra-Duty Boxes ........................................................................................... 14-15 Heavy-Duty Boxes ......................................................................................... 16-17 Extended Length Boxes .............................................................................. 18-21 DunnageReady Boxes .................................................................................22-23 Agricultural Boxes ....................................................................................... 24-25 Bulk Box Accessories ........................................................................................26 Hand-Held Containers ...............................................................................27 -

United States Patent (19) 11 Patent Number: 5,711,839 Dronzek, Jr
USOO571 1839A United States Patent (19) 11 Patent Number: 5,711,839 Dronzek, Jr. 45 Date of Patent: Jan. 27, 1998 54 PROCESS FOR THE PRODUCTION OF IN 5.242,650 9/1993 Rackovan et al. ....... 264/509 LINE GRAWURE-PRNTED INMOLD 5,344,305 9/1994 McKillip ................................. 42.5/503 LABELED BLOW MOLDED CONTAINERS 5,435,963 7/1995 Rackovan et al. ...................... 264/509 75 Inventor: Peter J. Dronzek, Jr., Thornwood, N.Y. FOREIGN PATENT DOCUMENTS 0281701 9/1988 European Pat. Off.. 73) Assignee: NorthStar Print Group, Watertown, 1807766 6/1969 Germany. Wis. WO9309925 5/1993 WIPO 21 Appl. No.: 909,663 Primary Examiner Merrick Dixon Attorney, Agent, or Firm-Hedman, Gibson & Costigan, 22 Filed: Aug. 12, 1997 PC. Related U.S. Application Data 57 ABSTRACT 63 Continuation of Ser. No. 527,751, Sep. 13, 1995, abandoned, Polymeric sheets or rolls suitable for in-line gravure printing and forming at high rates of production of in-mold labeled (51 int. Cl. B32B 3100 plastic containers comprise a polymeric transparent, trans 52 U.S. Cl. ............... 156/277; 156/244.11: 156/244.16; lucent or contact clear substrate having a thickness in the 264/132; 264/159; 264/239 range of 0.002 to 0.008 inches which is reverse gravure 58 Field of Search ................................ 264/132, 516, printed and gravure overcoated on the container-facing side 264/509, 510, 159, 513, 171,210.5, 235.6, with a heat activatable adhesive and gravure coated on the 244.11, 346, 151, 169,239, 241, DIG. 1; opposite side with an antistatic coating. -

The PMC 1002P Plastic Container Forming Machine
New performance features for The PMC 1002P the fastest, easiest plastic container production ever: Plastic Container Forming Machine • Variable Speed Drive with PLC Controls Quickly changes machine speed to adjust to material changes and new tooling sizes High Speed Forming Across the • User Friendly Touch Screen Display Full Range of Tapered or Provides direct control of machine operation, and Straightwall Plastic Containers monitoring of diagnostic and production data • Cam Actuated Machine & Station Functions Ensure synchronized operation and efficient, repeatable container production • Auto Start Function Reduces waste, improves efficiency and operator safety 6.50˝ Max. • Cycle Stop Function (165.1 mm) Automatically clears machine of blanks before shutting down to improve production efficiency and reduce waste • Electric Hot Air Heat Sealing 1.50˝ Min. Produces dependable, leak-resistant seals (38.1 mm) • Splice Detect Bottom Feeder Skips splices in bottom roll stock to improve tooling life 1.75˝ Min. and machine uptime efficiency (44.5 mm) 3.00˝ Max. • Incline or Horizontal Sidewall Blank Magazine (76.2 mm) Allows footprint to suit space and ergonomic needs 4.00˝ Max. (101.6 mm) • Servo Driven Bottom Feeder Reduces waste, improves efficiency, and allows use of printed bottom materials • Electrically Interlocked Safety Enclosure 6.68˝ Max. Allows production only when in the closed position 2.12˝ Min. (169.7 mm) (53.8 mm) • Machine Reverse 1.50˝ Min. Allows operators to clear material jams (38.1 mm ) • Quick Height Change Tooling Allows -

Horticultural Evaluation of Zein-Based Bioplastic Containers Matthew Ts Even Helgeson Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2009 Horticultural evaluation of zein-based bioplastic containers Matthew tS even Helgeson Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Horticulture Commons Recommended Citation Helgeson, Matthew Steven, "Horticultural evaluation of zein-based bioplastic containers" (2009). Graduate Theses and Dissertations. 10554. https://lib.dr.iastate.edu/etd/10554 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Horticultural evaluation of zein-based bioplastic containers by Matthew Steven Helgeson A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Horticulture Program of Study Committee: William R. Graves, Major Professor Richard J. Gladon David Grewell Iowa State University Ames, Iowa 2009 Copyright © Matthew Steven Helgeson, 2009. All rights reserved. ii TABLE OF CONTENTS ABSTRACT iii CHAPTER 1. GENERAL INTRODUCTION 1 Introduction 1 Thesis Organization 2 Literature Review 2 Literature Cited 8 CHAPTER 2. DEGRADATION AND NITROGEN RELEASE OF ZEIN-BASED 11 BIOPLASTIC CONTAINERS Abstract 11 Significance to the Nursery Industry 12 Introduction 13 Materials and Methods 15 Results and Discussion 18 Literature Cited 24 CHAPTER 3. ZEIN-BASED BIOPLASTIC CONTAINERS ALTER ROOT-ZONE 31 CHEMISTRY AND GROWTH OF GERANIUM Abstract 31 Significance to the Nursery Industry 32 Introduction 33 Materials and Methods 35 Results and Discussion 40 Literature Cited 47 CHAPTER 4. -

The Hard Facts About Plastic
THE HARD FACTS ABOUT PLASTIC The triangle symbols found on the underside of plastic containers do NOT mean the product is recyclable. These triangle symbols are the resin identification code, which indicates the type of plastic the product is made of. It does not mean that the item is recyclable. There is a disconnect between the manufacturers and the solid waste industry, which causes misinformation and confusion among the general public. Plastics #1 & 2 are readily recyclable and are usually recycled in the USA. These plastics are mostly converted into new plastic bottles or containers, while some are converted into textiles or insulation. Plastic #1 (PET) from the RethinkWaste service area stays in domestic markets; it is currently sent to Custom Polymers in Alabama and DAK in Indiana. Plastic #2 (HDPE) from the RethinkWaste service area is recycled through both domestic and international Figure 1: Plastic #1 (PET) Bottle Figure 2: Plastic #2 (HDPE) Container markets. When it is exported, it is sent to South Korea and Taiwan. When it stays domestic, it is sent to Talco Plastics and Epic Plastics in California. Plastics #3 - 7 are all versions of plastic which are difficult to recycle, and currently have no market. Prior to March of 2018, the United States sent large amounts of plastics and paper to China1 to be recycled. When China implemented the National Sword Policy, the accepted contamination rates changed from 5% to 0.5%. No facility in the United States or other industrialized countries could meet this new standard. In response, the flow of recyclable materials was diverted to secondary markets in different East and South East Asian countries. -

Packaging Guidelines for UN 3373 Shipments
Packaging Guidelines for UN 3373 Shipments Follow these instructions for packaging, marking and labeling Biological Substance, Category B (UN 3373) shipments for FedEx Express® services. Requirements for Biological Substance, to withstand, without leakage, an internal pressure Category B (UN 3373) Shipments differential of not less than 95 kPa in the range of -40 C to 55 C (-40 F to 130 F). To prevent contact between This guide outlines the requirements for shipping with multiple fragile primary receptacles, individually wrap or FedEx Express. In addition, all shipments must comply separate them inside the secondary container. with all applicable local, state and federal laws governing packing, marking and labeling. Blood, urine, fluids, and 4. Sturdy outer packaging. Use rigid outer packaging other specimens containing or suspected of containing constructed of corrugated fiberboard, wood, metal or infectious substances must be shipped according to plastic, appropriately sized for the contents. Chipboard applicable government, International Air Transport or paperboard boxes are unacceptable outer packaging. Association (IATA) and International Civil Aviation Limit the total volume for liquid samples to 4 L and Organization (ICAO) regulations. the total weight of dried samples to 4 kg per outer container. The minimum outer container size in the Customers who ship Biological Substance, Category B smallest overall external dimension is 4". Completed (UN 3373) shipments must comply with local, state and packages must be able to withstand a 4' (1.2-m) federal laws governing identification, classification, impact test as outlined in IATA 6.6.1 regulations. packaging and package markings (which may be in label Before sealing the outer packaging, you must make form).