Nanosilver-Enabled Food Storage Containers: a Case Study in Sustainability
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

25 Rating Food Storage Altfpa
RATINGS GOOD: FAIR: POOR: RATING FOOD STORAGE CONTAINERS Brand Testing Criteria Testers’ Comments HIGHLY RECOMMENDED Tupperware Rock ’N Serve Medium PRICE: $12.99 TESTERS’ COMMENTS: Well designed (handles, flat lid, vent for Deep Container PERFORMANCE, NEW: microwaving), with performance almost to match. We’re con- PERFORMANCE, USED: cerned about durability, though, as cracks developed during 100 MATERIAL: Plastic/polycarbonate DESIGN: dishwasher cycles. These cracks caused leaking during a second DISHWASHER INSTRUCTIONS: None submersion test. Some testers found it tricky to seal at first. SPECIAL NOTES: Open vent in lid to microwave Rubbermaid Stain Shield Square Container PRICE: $4.99 TESTERS’ COMMENTS: Handsome, sturdy container with wide rim PERFORMANCE, NEW: for easy handling. Lives up to marketing claims of stain resistance. MATERIAL: Plastic/polycarbonate-derived resin PERFORMANCE, USED: Odor control performance not perfect, though. DISHWASHER INSTRUCTIONS: Top rack only DESIGN: SPECIAL NOTES: Vent lid to microwave Genius VakSet: Four Vacuum PRICE: $57.99 TESTERS’ COMMENTS: Impressive performance comes with some Storage Containers with Pump PERFORMANCE, NEW: caveats: hassle factor of extra pumping step; vacuum-release PERFORMANCE, USED: method, which some may find unintuitive; containers rendered MATERIAL: Plastic/polycarbonate DESIGN: essentially useless if pump is lost. DISHWASHER INSTRUCTIONS: None SPECIAL NOTES: Lid cannot be microwaved Rubbermaid Seal’n Saver PRICE: $4.99 TESTERS’ COMMENTS: Staining was only problem encountered in PERFORMANCE, NEW: this sturdy container with excellent seal. MATERIAL: Plastic/polypropylene PERFORMANCE, USED: DISHWASHER INSTRUCTIONS: Top rack only DESIGN: SPECIAL NOTES: None RECOMMENDED WITH RESERVATIONS Betty Crocker Servables Container PRICE: $3.99 TESTERS’ COMMENTS: Decent, easy-handling container that didn’t PERFORMANCE, NEW: stand up too well to submersion and staining tests. -

Food Inspection Report Dated 9/10/20 Have Been Corrected
FOOD ESTABLISHMENT INSPECTION FORM FACILITY NAME DATE TIME IN TIME OUT Si Bon 9/17/2020 10:15 AM 11:00 AM ADDRESS FACILITY DESCRIPTION 40101 Monterey Ave #E5, Rancho Mirage, CA 92270 Not Applicable PERMIT HOLDER EMAIL Caupain LLC [email protected] Major Violations 0 PERMIT # EXPIRATION DATE SERVICE REINSPECTION DATE FACILITY PHONE # PE DISTRICT INSPECTOR NAME PR0061033 03/31/2021 Follow-up inspection Next Routine (760)837-0011 3621 0026 Darrel Balancier Points Deducted 4 The conditions listed below correspond to violations of the California Health and Safety Code and/or Riverside County Ordinances and must be corrected as indicated by the enforcement officer. The Department of Environmental Health appreciates your cooperation. Failure to correct listed violation(s) prior to the designated compliance date may necessitate an additional reinspection at a charge determined by Riverside County Ordinance 640. Major / Minor Violations:Major Violations are those that pose an imminent risk to public health and warrant immediate closure of the food facility or immediate correction. Minor Violations are those violations that do not pose an imminent public health risk, but do warrant correction. A GRADE REPRESENTS THE FOLLOWING RANGES: A = 100-90 Passed inspection / meets minimum health standards. B = 89-80 Did not pass inspection / does not meet minimum health standards. C = 79-0 Failed inspection / conditions exist which may pose a potential or actual threat to public health and safety. The SCORE 96 facility is required to display a grade card in a conspicuous place selected by the Enforcement Officer. The grade card shall not be concealed and can only be removed or relocated by the Enforcement Officer per County Ordinance 492 / County Code Section 8.40.020 In = In compliance Ë COS = Corrected on-site N/O = Not observed N/A = Not applicable OUT = Out of compliance DEMONSTRATION OF KNOWLEDGE COS MAJ OUT FOOD FROM APPROVED SOURCES COS MAJ OUT In N/A 1. -

224.50-585 Plastic Resin Code Labeling. (1) As Used in This Section: (A) "Rigid Plastic Container" Means Any Formed
224.50-585 Plastic resin code labeling. (1) As used in this section: (a) "Rigid plastic container" means any formed or molded article comprised predominantly of plastic resin and having a relatively inflexible finite shape or form intended primarily as a single service container with a capacity of eight (8) ounces or more and less than five (5) gallons; (b) "Rigid plastic bottle" means any rigid plastic container with a neck that is smaller than the container body with a capacity of sixteen (16) ounces or more and less than five (5) gallons; and (c) "Label" means a code label described in this section molded into the bottom of the plastic product. (2) All rigid plastic bottles and rigid plastic containers sold in Kentucky on and after January 1, 1992, shall be labeled with a code which indicates the resin used to produce the rigid plastic bottle or rigid plastic container. The code shall consist of a number placed inside a triangle and letters placed below the triangle. The triangle shall be equilateral, formed by three (3) arrows with the apex of each point of the triangle at the midpoint of each arrow, rounded with a short radius. The pointer or arrowhead of each arrow shall be at the midpoint of each side of the triangle with a short gap separating the pointer from the base of the adjacent arrow. The triangle, formed by the three (3) arrows curved at their midpoints, shall depict a clockwise path around the code number. The numbers and letters used shall be as follows: 1 = PETE (polyethylene terephthalate) 2 = HDPE (high density polyethylene) 3 = V (vinyl) 4 = LDPE (low density polyethylene) 5 = PP (polypropylene) 6 = PS (polystyrene) 7 = OTHER (represents all other resins, including layered plastics of a combination of materials). -

Plastic Bottles Today Innovating to Reach Today’S Consumer
PLASTICS MARKET WATCH PLASTIC BOTTLES TODAY INNOVATING TO REACH TODAY’S CONSUMER BETTER INDUSTRY. BETTER WORLD. February 2017 The Plastics Industry Association (PLASTICS) sends special thanks to the Brand Owners, Processors and Equipment Councils, and Rigid Plastics Packaging Group (RPPG) for their guidance and input on this Bottling Plastics Market Watch Report. Materials were compiled, written and edited by William (Bill) Mashek, with editorial assistance from Kim Holmes, George Southworth, Kendra Martin and Ashley Stoney at PLASTICS. Copyright Plastics Industry Association. 02 Plastics Market Watch—Watching: Bottling Plastics Market Watch Plastic Bottles Today Innovating to Reach Today’s Consumer A series examining the business of plastics, including demographics, economics, policy developments and technological trends in specific plastics end markets. Contents 05 Forward 08 Introduction: Plastic Bottles Today— Innovating to Reach Today’s Consumer 11 Role of Plastics in Bottling 16 Growth of Plastics Bottling 20 Innovation 25 Plastic Bottle Economics 29 Bottling Equipment & Machinery 31 Recycling Progress 37 Conclusion 42 Plastic Bottle Glossary 49 Sources 52 Plastics Market Watch Snapshot Plastics Market Watch—Watching: Bottling 03 04 Plastics Market Watch—Watching: Bottling Forward The Plastics Industry Association’s (PLASTICS’) Plastics Market Watch reports provide forward-looking data and insights on key plastics industry end markets. The series examines the business of plastics, including demographics, economics, policy developments and technological improvements for markets including automotive and transportation, healthcare and medical devices, packaging, building and construction, automotive recycling, bioplastics and consumer electronics. Given the role that plastics play in today’s modern society, Plastics Market Watch reports offer a holistic picture of our technology—from beginning-of-life resins and polymers to end-of-life management and recycling efforts. -

Why Can't All Plastics Be Recycled?
Why can’t all plastics be recycled? Plastic has become prevalent because it is inexpensive and it can be engineered with a wide range of properties. Plastics are strong but lightweight, somewhat resistant to being degraded by chemicals, sunlight, and bacteria, and are thermally and electrically insulating. What does the symbol mean? A chasing arrows symbol, or resin code, does not mean that a plastic container is recyclable. Most plastic containers are marked with the chasing arrows symbol ‐ number one through seven in the center. The number inside the arrows signifies the main chemical compound used to make that plastic container. Unfortunately, the symbol does not mean that plastic container can be recycled. There are seven resin codes used inside the chasing arrow symbols: 1. PETE Polyethylene Terephthalate is in pop and water bottles. Please recycle. 2. HDPE High Density Polyethylene is opaque and usually in bottles that store laundry detergent and milk. These are usually recyclable. 3. V Polyvinyl Chloride (PVC) is found in plastic pipes, shrink wrap. 4. LDPE Low Density Polyethylene is in produce bags, plastic wrap, and plastic bags. 5. PP Polypropylene is used for yogurt tubs, ketchup bottles. 6. PS Polystyrene is found in Styrofoam, used for egg crates. 7. Other This category covers a vast mixture of resins and includes food containers (clam shells), polycarbonate used in sport bottles, and bio‐based plastic used in compostable food containers. Why don’t we recycle all these plastics? Most cities collect #1 and #2 types of plastic, or the plastic bottles made from PETE/PET and HDPE resin. -
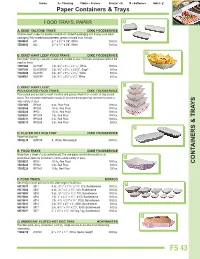
CONTAINERS & TRA YS Paper Containers & Trays
Paper Containers & Trays FOOD TRAYS, PAPER A A. DIXIE® VELTONE TRAYS DIXIE FOODSERVICE Shallow depth makes for excellent individual sandwich packaging and display case meat packaging. Poly-coated board prevents grease or liquid soak through. 1 1 15000641 641 3 /4'' x 5 /4'' x 7/8'', White 1000/cs. 1 1 15000642 642 3 /2'' x 7 /2'' x 5/8'', White 1000/cs. B B. DIXIE® KANT LEEK® FOOD TRAYS DIXIE FOODSERVICE Kant Leek® food trays are poly-coated and durable to resist moisture and grease without the need for liners. 1 11 19 15001666 KL100W 1 lb., 6 /4'' x 4 /16'' x 1 /32'', White 1000/cs. 3 13 ™ 15001595 KL300SAGE 3 lb., 8 /8'' x 5 /16'' x 23/32'', Sage 500/cs. 3 13 3 15004020 KL300W 3 lb., 8 /8'' x 5 /16'' x 2 /32'', White 500/cs. 5 1 9 15000051 KL500W 5 lb., 9 /16'' x 6 /8'' x 2 /32'', White 500/cs. C C. DIXIE® KANT LEEK® POLY-COATED FOOD TRAYS DIXIE FOODSERVICE Poly-coated and durable to resist moisture and grease. Made from a sheet of clay-coated board. The one-piece construction results in an economical open-top container to serve a wide variety of uses. 15001605 RP408 6 oz., Red Plaid 1000/cs. 15000456 RP258 1/4 lb., Red Plaid 1000/cs. 15005220 RP50 1/2 lb., Red Plaid 1000/cs. 15005204 RP1008 1 lb., Red Plaid 1000/cs. 15005211 RP2008 2 lb., Red Plaid 1000/cs. 15005212 RP3008 3 lb., Red Plaid 500/cs. -
Pet Food Storage and Automated Feeder
Pet Food Storage and Automated Feeder by RYAN HALL Submitted to the MECHANICAL ENGINEERING TECHNOLOGY DEPARTMENT In Partial Fulfillment of the Requirements for the Degree of Bachelor of Science In MECHANICAL ENGINEERING TECHNOLOGY at the College of Applied Science University of Cincinnati May 2007 © ...... RyanHall The author hereby grants to the Mechanical Engineering Technology Department permission to reproduce and distribute copies of the thesis document in whole or in part. Signature of Author hanical Engineering Technology Certified by Kettil Cedercreutz, Thesis Advisor Accepted by ~~~-L~Dr. Muthar Al-Ubaidi, Department Head Mechanical Engineering Technology ABSTRACT The problem addressed in this technical report is the lack of an effective system for feeding the family pet. The basic system for completing this task is to have a pet food storage container, to hold the food until feeding, and having a bowl for the food which is filled by the pet owner as a chore designated for a specific person in the family. There are some distinct flaws with this system. Some of which are that the food storage container is unsightly and it takes up too much space, and the task of feeding the pet has become cumbersome and sometimes goes undone (or is done too much due to a lack of communication.) In order to eliminate this problem a Pet Food Storage and Automated Feeder was developed in order to store all pet food up and out of the way while being able to feed the pet with little or no effort and at programmable times. Many different steps were taken in the design and development of this project. -

PRUDENT FOOD STORAGE: Questions & Answers
Version 4.0 Updated December 2003 Supersedes Version 3.50 PRUDENT FOOD STORAGE: Questions & Answers Alan T. Hagan Author of The Prudent Pantry: Your Guide to Building a Food Insurance Program "In this work, when it shall be found that much is omitted, let it not be forgotten that much likewise is performed." Samuel Johnson, 1775, upon completion of his dictionary. Courtesy of James T. Stevens ACKNOWLEDGEMENTS: Diana Hagan, my wife, for endless patience in the years since I created this FAQ; Susan Collingwood for sage advice; Lee Knoper; BarbaraKE; Gary Chandler; Skipper Clark, author of Creating the Complete Food Storage Program; Denis DeFigueiredo; Al Durtschi for resources and encouragement; Craig Ellis; Pyotr Filipivich; Sandon A. Flowers; Amy Gale, editor of the rec.food.cooking FAQ; Geri Guidetti, of the Ark Institute; Woody Harper; Higgins10; Robert Hollingsworth; Jenny S. Johanssen; Kahless; James T. Stevens, author of Making The Best of Basics; Amy Thompson (Saco Foods); Patton Turner; Logan VanLeigh; Mark Westphal; Rick Bowen; On-Liner and The Rifleman in the UK; Myal in Australia; Rosemarie Ventura; Rex Tincher; Halcitron; Noah Simoneaux; a number of folks who for reasons sufficient unto themselves wish to remain anonymous; and last, but certainly not least, Leslie Basel, founding editor of the rec.food.preserving FAQ, without whom I'd never have attempted this in the first place. The home of the Prudent Food Storage FAQ can be found at: http://athagan.members.atlantic.net/Index.html Check there to be sure of the most current FAQ version. Updated: 9/18/96; 4/16/97; 7/21/97; 10/20/97; 9/15/98; 11/02/99; 12/01/03 Copyright ã 1996, 1997, 1998, 1999, 2003. -

Reusable Plastic Container Fact Sheet
Reusable Plastic Container Fact Sheet Reusable Plastic Containers The use of reusable plastic containers(RPC’s) by fruit and vegetable growers has recently become more popular as the push for sustainability increases. As more producers become concerned with decreasing the amount of waste created throughout the supply chain, sustainable options become progressively more relevant. The movement away from corrugated cardboard as the primary containment through transportation and distribution has become a viable RPC Companies option as growers look to RPC There are several different companies companies for a multi-purpose, more that produce and distribute quality sustainable option. RPCs. (Companies not limited to list.) Sustainable Transport Packaging RPCs can be used more successfully Their headquarters are located in St. in different application than Petersburg, FL. with various stocking corrugated cardboard throughout the locations around the U.S. They are supply chain. Growers are able to use a reseller, distributor, and custom RPCs from harvesting the product, manufacturer of various RPCs for a to transportation, to distribution number of applications. and sales as the display making Rehrig Pacific Company their operation more efficient. With their U.S. corporate headquarters This efficiency paired with the Corrugated Cardboard in Los Angeles, CA Rehrig has been sustainability of a product that lasts • Environmental Protection serving the Agriculture industry with for multiple uses, is the very option Agency (EPA) Estimates 30.1 RPCs since the 1980’s. Their products producers are searching for. Million tons of corrugated are designed to help producers safely cardboard are generated each and effectively transport products to RPCs have been around for more year(1) desired markets. -

A COMPARISON of GELATIN and TRADITIONAL PLASTICS Grant Proposal
A COMPARISON OF GELATIN AND TRADITIONAL PLASTICS Grant Proposal Team H: Allison Brookhart, Bao Le, Amelia Navarre, Kelly Simpson, Ame Tsamasse PROJECT SUMMARY To what degree would gelatin plastics be a viable option compared to conventional plastics in the longevity of the plastic function, biodegradability, cleanliness, and in economic feasibility? If gelatin plastics last longer, are more biodegradable, are healthier for humans, and are inexpensive, then they would be a feasible alternative to conventional plastics. Plastic waste and microplastics are a major problem that could have detrimental effects on marine ecosystems, terrestrial animal species, human health, and more. BACKGROUND In Case Study 1, we found there was not a lot of research on gelatin plastics with a hydrophobic coating. We wanted to create gelatin plastics that can do well in moist environments, so as to be suited for the foodservice industry. In the first case study, we created three different coatings for the plastics we made. The coating with beeswax and lignin mixed acetone fared the best out of the three: it was the most hydrophobic and adhered to the plastic the longest. In this case study, we want to continue this research. The questions we are now posting are the unknowns that arose from the previous experiment. This includes asking if there is a way to make a better film that holds for a longer time period and testing the efficiency of the biodegradability of the plastics. Gelatin plastic has long been explored as a safer and more biodegradable product for food packaging. It is a more environmentally-friendly alternative to the plastic used today and could serve as a possible solution to the global plastic epidemic (Hanani et al., 2014). -

Download Catalog
DELIVERING VALUE BEYOND THE BOX BUCKHORN REUSABLE PACKAGING SOLUTIONS Reusable Packaging Solutions Product Catalog PRODUCT CATALOG Table of Contents Buckhorn Inc. Why Buckhorn .......................................................................................... 3-6 400 TechneCenter Drive, Suite 215 Bulk Boxes ..................................................................................................... 7 Milford, OH 45150 Bulk Boxes Overview ....................................................................................... 8-9 Toll Free: (800) 543-4454 General Purpose Boxes ................................................................................ 10-11 Tel: (513) 831-4402 Fax: (513) 831-5474 Standard-Duty Boxes ....................................................................................12-13 Extra-Duty Boxes ........................................................................................... 14-15 Heavy-Duty Boxes ......................................................................................... 16-17 Extended Length Boxes .............................................................................. 18-21 DunnageReady Boxes .................................................................................22-23 Agricultural Boxes ....................................................................................... 24-25 Bulk Box Accessories ........................................................................................26 Hand-Held Containers ...............................................................................27 -

United States Patent (19) 11 Patent Number: 5,711,839 Dronzek, Jr
USOO571 1839A United States Patent (19) 11 Patent Number: 5,711,839 Dronzek, Jr. 45 Date of Patent: Jan. 27, 1998 54 PROCESS FOR THE PRODUCTION OF IN 5.242,650 9/1993 Rackovan et al. ....... 264/509 LINE GRAWURE-PRNTED INMOLD 5,344,305 9/1994 McKillip ................................. 42.5/503 LABELED BLOW MOLDED CONTAINERS 5,435,963 7/1995 Rackovan et al. ...................... 264/509 75 Inventor: Peter J. Dronzek, Jr., Thornwood, N.Y. FOREIGN PATENT DOCUMENTS 0281701 9/1988 European Pat. Off.. 73) Assignee: NorthStar Print Group, Watertown, 1807766 6/1969 Germany. Wis. WO9309925 5/1993 WIPO 21 Appl. No.: 909,663 Primary Examiner Merrick Dixon Attorney, Agent, or Firm-Hedman, Gibson & Costigan, 22 Filed: Aug. 12, 1997 PC. Related U.S. Application Data 57 ABSTRACT 63 Continuation of Ser. No. 527,751, Sep. 13, 1995, abandoned, Polymeric sheets or rolls suitable for in-line gravure printing and forming at high rates of production of in-mold labeled (51 int. Cl. B32B 3100 plastic containers comprise a polymeric transparent, trans 52 U.S. Cl. ............... 156/277; 156/244.11: 156/244.16; lucent or contact clear substrate having a thickness in the 264/132; 264/159; 264/239 range of 0.002 to 0.008 inches which is reverse gravure 58 Field of Search ................................ 264/132, 516, printed and gravure overcoated on the container-facing side 264/509, 510, 159, 513, 171,210.5, 235.6, with a heat activatable adhesive and gravure coated on the 244.11, 346, 151, 169,239, 241, DIG. 1; opposite side with an antistatic coating.