Re-Evaluation Note REV2018-17
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COMBINED LIST of Particularly Hazardous Substances
COMBINED LIST of Particularly Hazardous Substances revised 2/4/2021 IARC list 1 are Carcinogenic to humans list compiled by Hector Acuna, UCSB IARC list Group 2A Probably carcinogenic to humans IARC list Group 2B Possibly carcinogenic to humans If any of the chemicals listed below are used in your research then complete a Standard Operating Procedure (SOP) for the product as described in the Chemical Hygiene Plan. Prop 65 known to cause cancer or reproductive toxicity Material(s) not on the list does not preclude one from completing an SOP. Other extremely toxic chemicals KNOWN Carcinogens from National Toxicology Program (NTP) or other high hazards will require the development of an SOP. Red= added in 2020 or status change Reasonably Anticipated NTP EPA Haz list COMBINED LIST of Particularly Hazardous Substances CAS Source from where the material is listed. 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide Acutely Toxic Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- Acutely Toxic 1-(2-Chloroethyl)-3-(4-methylcyclohexyl)-1-nitrosourea (Methyl-CCNU) Prop 65 KNOWN Carcinogens NTP 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) IARC list Group 2A Reasonably Anticipated NTP 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) (Lomustine) Prop 65 1-(o-Chlorophenyl)thiourea Acutely Toxic 1,1,1,2-Tetrachloroethane IARC list Group 2B 1,1,2,2-Tetrachloroethane Prop 65 IARC list Group 2B 1,1-Dichloro-2,2-bis(p -chloropheny)ethylene (DDE) Prop 65 1,1-Dichloroethane -

Liquid and Gas Chromatographic Multi-Residue Pesticide Determination in Animal Tissues
Pestic. Sci. 1997, 49,56È64 Liquid and Gas Chromatographic Multi-residue Pesticide Determination in Animal Tissues Aurora Navas Dj az,* Angeles Garcj a Pareja & Francisco Garcj aSanchez Departamento de Qu•mica Anal•tica, Facultad de Ciencias, Universidad de Malaga, 29071-Malaga, Spain (Received 13 June 1995; revised version received 17 April 1996; accepted 16 August 1996) Abstract: A liquid chromatography multi-residue method with photometric detection has been developed. The method is applicable to the quantitative deter- mination of organochlorine (tetradifon, dicofol, chlorfenson, chlorobenzilate), organophosphorus (fenitrothion, azinphos-ethyl) and carbamate (pirimicarb) pesticides in animal tissues. The extracted residues are cleaned up by gel- permeation chromatography. A further fractionation on silica Sep-Pack car- tridges is included in the procedure. A gas chromatographic method with electron-capture detection for the analysis of the same pesticides was carried out and the results in the two cases compared. Lower detection and quantitation limits and similar recoveries of pesticides from spiked pig liver and brain samples were obtained by the LC method. Key words: liquid chromatography, gas chromatography, multi-residue pesti- cides, animal tissues 1 INTRODUCTION by various element-sensitive detectors, leading to reli- ance on mass spectrometry because of its ability to help Pest control in modern agriculture includes treatment to deÐne the structure. This has especially been true in of crops pre- and post-harvest with a variety of chemi- the case of the combination of gas chromatography cals, such as herbicides and insecticides in the pre- with mass spectrometry (GC/MS). One of the obvious harvest stage and with fungicides and rodenticides in Ðelds of application for this technique was the analysis the storage stage of the total harvest process. -

Spatial Variation in Non-Target Effects of the Insecticides Chlorpyrifos
Pesticide Science Pestic Sci 55:875±886 (1999) Spatial variation in non-target effects of the insecticides chlorpyrifos, cypermethrin and pirimicarb on Collembola in winter wheat Geoffrey K Frampton* Biodiversity and Ecology Division, School of Biological Sciences, University of Southampton, Bassett Crescent East, Southampton, SO16 7PX, UK Abstract: Contiguous winter wheat ®elds of similar cropping history and soil type were used in a study of the responses of Collembola to summer sprays of cypermethrin and pirimicarb in southern England. Chlorpyrifos was included in the study as a toxic standard. Epigeic arthropods were captured by suction sampling and crop-inhabiting species obtained by dissecting wheat ears. Eight genera of Collembola responded signi®cantly to the insecticide treatments. Collembolan abundance decreased after chlorpyrifos was applied but increased after use of cypermethrin. Negative effects of cypermethrin and pirimicarb on Collembola were not detected in this study. Effects of chlorpyrifos varied spatially as a result of faunal heterogeneity among the ®elds, despite apparent homogeneity of the site. Some species known to be susceptible to chlorpyrifos were absent from one or more of the ®elds. The implications of these ®ndings for the interpretation of non-target pesticide effects and the potential use of Collembola as bioindicators in ®eld studies with pesticides are discussed. # 1999 Society of Chemical Industry Keywords: insecticides; chlorpyrifos; cypermethrin; pirimicarb; Collembola; side-effects; bioindicators -

Gas-Phase Chemical Reduction (GPCR)
Gas-Phase Chemical Reduction (GPCR) Name of Process: Status: Gas-Phase Chemical Reduction (GPCR) A Commercial system operated in Australia for more than 5 years, treating Vendor: more than 2,500 tons of PCB’s, DDT and other POPs. In 1999 a full-scale test ELI Eco Logic International Inc. on HCB was conducted using the commercial plant. Web site: http://www.ecologic.ca Eco Logic’s partners in Japan have recently built a semi-mobile GPCR plant for Applicable Pesticides and related the treatment of PCB wastes, which will be operational in 2003. POPs wastes: Pesticides such as Hexachlorobenzene, In combination with Foster Wheeler and Kvaerner the company is DDT, Aldrin, Dieldrin, HCB’s, DDT, PCB’s, participating at present in the ACWA (Army Chemical Weapons Assessment) dioxins and furans and other POPs. Program for the destruction of chemical warfare agents. Eco Logic has partnered with Torftech Inc. for the treatment of soils and sediments at rates of up to 20 tons per hour. Eco Logic has also been selected by UNIDO for a pilot project for treatment of 1000 tons of PCB wastes in Slovakia. Additional approvals received: -for PCB and dioxin waste in Japan -for PCB’s TSCA permit in USA -for PCB’s and other toxic compounds in the Province of Ontario (Canada) Technology description: Eco Logic’s GPCR technology involves the gas-phase chemical reduction of organic compounds by hydrogen at a temperature of 850°C or higher. Chlorinated hydrocarbons, such as HCB, polychlorinated dibenzo-p-dioxins (dioxins) and other POPs, are chemically reduced to methane and hydrogen chloride (HCl). -

How to Reduce Bee Poisoning from Pesticides
PNW 591 December 2006 How to ReduceReduce BeeBee PoisoningPoisoning from pesticides H. Riedl E. Johansen L. Brewer J. Barbour A Pacific Northwest Extension publication Oregon State University • University of Idaho • Washington State University Contents Pollinators are essential to Pacific Northwest agriculture .......................................................................1 Rules to protect bees ..............................................................................................................................1 Causes of bee poisoning in the Pacific Northwest .................................................................................2 Investigating a suspected bee poisoning ................................................................................................2 Signs and symptoms of bee poisoning ...................................................................................................2 Honey bees .................................................................................................................................................... 2 Managed solitary bees ................................................................................................................................... 3 Ways to reduce bee poisoning ...............................................................................................................3 Beekeeper–grower cooperation ..................................................................................................................... 3 What pesticide -

Effects of Bendiocarb Insecticide on Lipid Peroxidation, Antioxidant Enzymes and DNA Damage in Human Leukocytes
Erciyes Üniversitesi Erciyes University Fen Bilimleri Enstitüsü Dergisi Journal of Institue Of Science and Cilt 37, Sayı 2 , 2021 Technology Volume 37, Issue 2, 2021 Effects of Bendiocarb Insecticide on Lipid Peroxidation, Antioxidant Enzymes and DNA Damage in Human Leukocytes İsrafil DOĞANYİĞİT1 , Fatma ÖZTÜRK KÜP2* 1Erciyes University, Graduate School of Natural and Applied Sciences, Kayseri, TURKEY 2*Erciyes University, Faculty of Science, Department of Biology, Kayseri, TURKEY (Alınış / Received: 31.08.2020, Kabul / Accepted: 31.08.2021, Online Yayınlanma / Published Online: 31.08.2021) Keywords Abstract: In this study, we aimed to determine the toxic effect of increasing doses of Bendiocarb, Bendiocarb, an insecticide from the carbamate group, on human blood cells in vitro via Genotoxicity, by Comet test, determination of total antioxidant capacity, antioxidant enzyme activity Comet Assay, and malondialdehyde (MDA) level. Leukocyte isolation was performed from blood Pesticides, samples taken from six healthy male individuals who did not smoke and use alcohol and Antioxidant Enzymes were not exposed to any chemicals in the working environment, and then their leukocytes exposed to increasing doses of Bendiocarb (20, 40, 80, 160 µg / mL). Comet test, iron reduction antioxidant capacity (FRAP), 2-2'azinobis 3-ethylbenzothiazoline-6- sulfonic acid (ABTS/TEAC) analysis with superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and MDA were evaluated. At the end of the study, it was determined that antioxidant enzyme levels decreased significantly due to dose increase, whereas MDA levels increased significantly in all doses (p<0.05). According to FRAP and TEAC methods, the antioxidant capacity of blood cells decreased significantly in increasing doses of Bendiocarb compared to the control group (p<0.05). -
Method Description
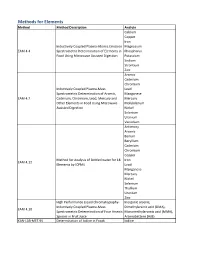
Methods for Elements Method Method Description Analyte Calcium Copper Iron Inductively Coupled Plasma-Atomic Emission Magnesium EAM 4.4 Spectrometric Determination of Elements in Phosphorus Food Using Microwave Assisted Digestion Potassium Sodium Strontium Zinc Arsenic Cadmium Chromium Inductively Coupled Plasma-Mass Lead Spectrometric Determination of Arsenic, Manganese EAM 4.7 Cadmium, Chromium, Lead, Mercury and Mercury Other Elements in Food Using Microwave Molybdenum Assisted Digestion Nickel Selenium Uranium Vanadium Antimony Arsenic Barium Beryllium Cadmium Chromium Copper Method for Analysis of Bottled water for 18 Iron EAM 4.12 Elements by ICPMS Lead Manganese Mercury Nickel Selenium Thallium Uranium Zinc High Performance Liquid Chromatography- Inorganic arsenic, Inductively Coupled Plasma-Mass Dimethylarsinic acid (DMA), EAM 4.10 Spectrometric Determination of Four Arsenic Monomethylarsonic acid (MMA), Species in Fruit Juice Arsenobetaine (AsB) KAN-LAB-MET.95 Determination of Iodine in Foods Iodine Methods for Radionuclides Method Method Description Analyte Determination of Strontium-90 in Foods by WEAC.RN.METHOD.2.0 Strontium-90 Internal Gas-Flow Proportional Counting Americium-241 Cesium-134 Cesium-137 Determination of Gamma-Ray Emitting Cobalt-60 WEAC.RN.METHOD.3.0 Radionuclides in Foods by High-Purity Potassium-40 Germanium Spectrometry Radium-226 Ruthenium-103 Ruthenium-106 Thorium-232 Methods for Pesticides/Industrial Chemicals Method Method Description Analyte Extraction Method: Analysis of Pesticides KAN-LAB-PES.53 and -

Interaction Profile for Mixture of Insecticides: Pyrethroids
44 5. References Abdel-Rahman A, Dechkovskaia AM, Goldstein LB, et al. 2004. Neurological deficits induced by malathion, DEET, and permethrin, alone or in combination in adult rats. J Toxicol Environ Health A 67(4):331-356. ATSDR. 1992. Public health assessment guidance manual. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry. ATSDR. 2003a. Toxicological profile for pyrethrins and pyrethroids. Agency for Toxic Substances and Disease Registry. http://www.atsdr.cdc.gov/ToxProfiles/tp155.pdf. March 29, 2013. ATSDR. 2004a. Guidance manual for the assessment of joint toxic action of chemical mixtures. Atlanta, GA: Agency for Toxic Substances and Disease Registry. http://www.atsdr.cdc.gov/interactionprofiles/IP-ga/ipga.pdf. April 2, 2013. ATSDR. 2004b. Interaction profile for benzene, toluene, ethylbenzene, and xylenes (BTEX). Agency for Toxic Substances and Disease Registry. http://www.atsdr.cdc.gov/interactionprofiles/IP- btex/ip05.pdf. March 29, 2013. Belden JB, Lydy MJ. 2006. Joint toxicity of chlorpyrifos and esfenvalerate to fathead minnows and midge larvae. Environ Toxicol Chem 25(2):623-629. Bosgra S, van Eijkeren JC, van der Schans MJ, et al. 2009. Toxicodynamic analysis of the inhibition of isolated human acetylcholinesterase by combinations of methamidophos and methomyl in vitro. Toxicol Appl Pharmacol 236(1):1-8. Burr SA, Ray DE. 2004. Structure-activity and interaction effects of 14 different pyrethroids on voltage- gated chloride ion channels. Toxicol Sci 77(2):341-346. Cao Z, Shafer TJ, Crofton KM, et al. 2011. Additivity of pyrethroid actions on sodium influx in cerebrocortical neurons in primary culture. -

Acephate Appendices
Acephate Appendices Appendix A: Ecological Effects Data............................................................................... 2 Appendix B: Aquatic Exposure Modeling Runs ............................................................ 28 Appendix C: Incident Database Information .................................................................. 52 Appendix D: ECOTOX Database, Accepted.................................................................. 56 Appendix D1: ECOTOX Database, Excluded.................................................................. 69 Appendix D2: ECOTOX Database, Mixtures .................................................................. 90 Appendix E: Toxicity Categories and LOCs .................................................................. 92 Appendix F: T-REX Model outputs ............................................................................... 93 Appendix F1: T-HERPS Model outputs......................................................................... 103 Appendix G: Label Information.................................................................................... 113 Appendix H: Mixture Product Names .......................................................................... 116 Appendix I: Fate Properities ........................................................................................ 118 Appendix J: Usage Information................................................................................... 124 Appendix K: GIS Maps ............................................................................................... -

REPORTS and STUDIES No
CL$ tMPk I IL)flO rIJIJLInr.L1 um I ru r4pI I iur WUNLUNtL T 1 WUIILL) IN I nNINA1 UNAL INTERNATIONAL ENVIRONMENT AGRICULTURE EDUCATIONAL ORGANIZATION METEOROLOGICAL MARITIME ATOMIC ENERGY PROGRAMME ORGANIZATION SCIENTIFIC AND ORGAN1ZATION ORGANZATrON AGENCY OF THE UNITED CULTURAL GENEVA NAIROBI NATI ONS ORGANIZATION GENE VA LONDON VIENNA ROME PARIS 9 () -, - IMO/FAO/IJNESCO/WMO/WHO/IAEA/UN/UNEP JOINT GROUP OF EXPERTS ON THE SCIENTIFIC ASPECTS OF MARINE POLLUTION - GESAMP - REPORTS AND STUDIES No. 35 The Evaluation of the Hazards of Harmful Substances Carried by Ships: Revision of GESAMP Reports and Studies No. 17 Oon INTERNATIONAL MARITIME ORGANIZATION P}/12 j Lfl Reports and Studies No.35 IMO/FAO/Unesco/WNO/WHO/IAEA/UN/UNEP Joint Group of Experts on the Scientific Aspects of Marine Pollution (GESAMP); THE EVALUATION OF THE HAZARDS OF HARMFUL SUBSTANCES CARRIED BY SHIPS; REVISION OF GESAMP REPORTS AND STUDIES NO.17 IMO, 1989 &t This report is an updated and revised version of an earlier report on tne evaluation of ttie uazards of marmfut substances carried by snips (CESAMP Reports and Studies No.17) wicn was published by 1110 in 1982. 967 SV / :i en NOTES I CESAMP is an advisory body consisting of specialized experts nominated by the sponsoring agencies (1140, FAO, Unesco, WMO, WHO, IAEA, UN, UNEP). Its principal task is to provide scientific advice on marine pollution problems to the sponsoring agencies and to the Intergovernmental. Oceanographic Commission (lOG). 2 This report is available in English from any of tue sponsoring agencies. 3 The report contains views expressed by members of GESAMP wuo act in their individual capacities; trieir views may not necessarily correspond with those of the sponsoring agencies. -

PDP Pesticide History Years Each Pesticide Was Reported from 1991 - 2020
USDA, AMS, S&T, MPD - Pesticide Data Program (PDP) 2 November 2020 PDP Pesticide History Years each pesticide was reported from 1991 - 2020 Pest Pesticide Name Code Years Pesticide was Reported 1,2,4-Triazole A68 2003 - 2007 1-Naphthol 382 1994 - 2000, 2003 - 2020 2,3,5-Trimethacarb A72 2020 2,4,5-T 312 2001 - 2016 2,4,5-TP AJE 2010 - 2013 2,4-D 026 1992 - 1998, 2002 - 2020 2,4-DB 317 1996 - 1997, 2003 - 2016, 2019 2,4-dimethyl aniline (2,4 DMA) AGQ 2007 - 2008 2,4-dimethylphenyl formamide (2,4-DMPF) AGR 2007 - 2012, 2016 - 2020 2,6-dichlorobenzamide 272 2010, 2017 2,6-DIPN AFZ 2016 - 2020 3,5-Dichloroaniline ABM 2003 3-Hydroxycarbofuran 512 1993 - 2020 3-ketocarbofuran 218 2001 - 2002 4,4-dibromobenzophenone AGS 2007 - 2008 4-Hydroxychlorothalonil ANM 2017 4-Hydroxydiphenylamine B19 1995 - 1997 5-Hydroxythiabendazole B28 1996 - 1997, 2003 - 2020 Abamectin 948 1994 - 1997, 1999, 2012 - 2020 Acephate 204 1991 - 2020 Acequinocyl AKS 2013 - 2015, 2020 Acetamiprid B80 2004 - 2020 Acetochlor 807 2001, 2003 - 2020 Acetochlor ethanesulfonic acid (ESA) ABN 2001 - 2013, 2017 Acetochlor oxanilic acid (OA) ABO 2001, 2003 - 2013 Acibenzolar S methyl B51 2003 - 2020 Acifluorfen 727 2003 - 2007, 2014 - 2017 Aclonifen D58 2017 - 2020 Acrinathrin A03 2012 - 2015, 2017 Afidopyropen F87 2020 Alachlor 227 1997 - 2020 Alachlor ethanesulfonic acid (ESA) ABP 2001 - 2013, 2017 Alachlor oxanilic acid (OA) ABQ 2001 - 2013 Aldicarb 167 1994 - 2020 Aldicarb sulfone 168 1994 - 2020 Aldicarb sulfoxide 169 1993 - 2020 Aldrin 001 1997 - 2020 Allethrin 002 1994, 1999 -

Research and Special Programs Admin., DOT § 172.101
Research and Special Programs Admin., DOT § 172.101 TABLE 2 TO APPENDIX AÐRADIONUCLIDESÐ the class of the material must be determined Continued in accordance with § 173.2a of this sub- chapter. 3. This appendix contains two columns. (2)Ð (3)ÐReportable Atomic The first column, entitled ``S.M.P.'' (for se- (1)ÐRadionuclide Num- Quantity (RQ) ber Ci (TBq) vere marine pollutants), identifies whether a material is a severe marine pollutant. If the Ytterbium-169 .......................... 70 10 (.37) letters ``PP'' appear in this column for a ma- Ytterbium-175 .......................... 70 100 (3.7) terial, the material is a severe marine pol- Ytterbium-177 .......................... 70 1000 (37) lutant, otherwise it is not. The second col- Ytterbium-178 .......................... 70 1000 (37) umn, entitled ``Marine Pollutant'' , lists the Yttrium-86 ................................ 39 10 (.37) Yttrium-86m ............................. 39 1000 (37) marine pollutants. Yttrium-87 ................................ 39 10 (.37) 4. If a material not listed in this appendix Yttrium-88 ................................ 39 10 (.37) meets the criteria for a marine pollutant, as Yttrium-90 ................................ 39 10 (.37) provided in the General Introduction of the Yttrium-90m ............................. 39 100 (3.7) IMDG Code, Guidelines for the Identification Yttrium-91 ................................ 39 10 (.37) of Harmful Substances in Packaged Form, Yttrium-91m ............................. 39 1000 (37) Yttrium-92 ................................ 39 100 (3.7) the material may be transported as a marine Yttrium-93 ................................ 39 100 (3.7) pollutant in accordance with the applicable Yttrium-94 ................................ 39 1000 (37) requirements of this subchapter. Yttrium-95 ................................ 39 1000 (37) 5. If approved by the Associate Adminis- Zinc-62 ..................................... 30 100 (3.7) trator for Hazardous Materials Safety, a ma- Zinc-63 ....................................