Visual Acuity in Pelagic Fishes and Mollusks
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

CHECKLIST and BIOGEOGRAPHY of FISHES from GUADALUPE ISLAND, WESTERN MEXICO Héctor Reyes-Bonilla, Arturo Ayala-Bocos, Luis E
ReyeS-BONIllA eT Al: CheCklIST AND BIOgeOgRAphy Of fISheS fROm gUADAlUpe ISlAND CalCOfI Rep., Vol. 51, 2010 CHECKLIST AND BIOGEOGRAPHY OF FISHES FROM GUADALUPE ISLAND, WESTERN MEXICO Héctor REyES-BONILLA, Arturo AyALA-BOCOS, LUIS E. Calderon-AGUILERA SAúL GONzáLEz-Romero, ISRAEL SáNCHEz-ALCántara Centro de Investigación Científica y de Educación Superior de Ensenada AND MARIANA Walther MENDOzA Carretera Tijuana - Ensenada # 3918, zona Playitas, C.P. 22860 Universidad Autónoma de Baja California Sur Ensenada, B.C., México Departamento de Biología Marina Tel: +52 646 1750500, ext. 25257; Fax: +52 646 Apartado postal 19-B, CP 23080 [email protected] La Paz, B.C.S., México. Tel: (612) 123-8800, ext. 4160; Fax: (612) 123-8819 NADIA C. Olivares-BAñUELOS [email protected] Reserva de la Biosfera Isla Guadalupe Comisión Nacional de áreas Naturales Protegidas yULIANA R. BEDOLLA-GUzMáN AND Avenida del Puerto 375, local 30 Arturo RAMíREz-VALDEz Fraccionamiento Playas de Ensenada, C.P. 22880 Universidad Autónoma de Baja California Ensenada, B.C., México Facultad de Ciencias Marinas, Instituto de Investigaciones Oceanológicas Universidad Autónoma de Baja California, Carr. Tijuana-Ensenada km. 107, Apartado postal 453, C.P. 22890 Ensenada, B.C., México ABSTRACT recognized the biological and ecological significance of Guadalupe Island, off Baja California, México, is Guadalupe Island, and declared it a Biosphere Reserve an important fishing area which also harbors high (SEMARNAT 2005). marine biodiversity. Based on field data, literature Guadalupe Island is isolated, far away from the main- reviews, and scientific collection records, we pres- land and has limited logistic facilities to conduct scien- ent a comprehensive checklist of the local fish fauna, tific studies. -

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

Order BERYCIFORMES ANOPLOGASTRIDAE Anoplogaster
click for previous page 2210 Bony Fishes Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths by J.R. Paxton iagnostic characters: Small (to 16 cm) Dberyciform fishes, body short, deep, and compressed. Head large, steep; deep mu- cous cavities on top of head separated by serrated crests; very large temporal and pre- opercular spines and smaller orbital (frontal) spine in juveniles of one species, all disap- pearing with age. Eyes smaller than snout length in adults (but larger than snout length in juveniles). Mouth very large, jaws extending far behind eye in adults; one supramaxilla. Teeth as large fangs in pre- maxilla and dentary; vomer and palatine toothless. Gill rakers as gill teeth in adults (elongate, lath-like in juveniles). No fin spines; dorsal fin long based, roughly in middle of body, with 16 to 20 rays; anal fin short-based, far posterior, with 7 to 9 rays; pelvic fin abdominal in juveniles, becoming subthoracic with age, with 7 rays; pectoral fin with 13 to 16 rays. Scales small, non-overlap- ping, spinose, cup-shaped in adults; lateral line an open groove partly covered by scales. No light organs. Total vertebrae 25 to 28. Colour: brown-black in adults. Habitat, biology, and fisheries: Meso- and bathypelagic. Distinctive caulolepis juvenile stage, with greatly enlarged head spines in one species. Feeding mode as carnivores on crustaceans as juveniles and on fishes as adults. Rare deepsea fishes of no commercial importance. Remarks: One genus with 2 species throughout the world ocean in tropical and temperate latitudes. The family was revised by Kotlyar (1986). Similar families occurring in the area Diretmidae: No fangs, jaw teeth small, in bands; anal fin with 18 to 24 rays. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Order MYCTOPHIFORMES NEOSCOPELIDAE Horizontal Rows
click for previous page 942 Bony Fishes Order MYCTOPHIFORMES NEOSCOPELIDAE Neoscopelids By K.E. Hartel, Harvard University, Massachusetts, USA and J.E. Craddock, Woods Hole Oceanographic Institution, Massachusetts, USA iagnostic characters: Small fishes, usually 15 to 30 cm as adults. Body elongate with no photophores D(Scopelengys) or with 3 rows of large photophores when viewed from below (Neoscopelus).Eyes variable, small to large. Mouth large, extending to or beyond vertical from posterior margin of eye; tongue with photophores around margin in Neoscopelus. Gill rakers 9 to 16. Dorsal fin single, its origin above or slightly in front of pelvic fin, well in front of anal fins; 11 to 13 soft rays. Dorsal adipose fin over end of anal fin. Anal-fin origin well behind dorsal-fin base, anal fin with 10 to 14 soft rays. Pectoral fins long, reaching to about anus, anal fin with 15 to 19 rays.Pelvic fins large, usually reaching to anus.Scales large, cycloid, and de- ciduous. Colour: reddish silvery in Neoscopelus; blackish in Scopelengys. dorsal adipose fin anal-fin origin well behind dorsal-fin base Habitat, biology, and fisheries: Large adults of Neoscopelus usually benthopelagic below 1 000 m, but subadults mostly in midwater between 500 and 1 000 m in tropical and subtropical areas. Scopelengys meso- to bathypelagic. No known fisheries. Remarks: Three genera and 5 species with Solivomer not known from the Atlantic. All Atlantic species probably circumglobal . Similar families in occurring in area Myctophidae: photophores arranged in groups not in straight horizontal rows (except Taaningichthys paurolychnus which lacks photophores). Anal-fin origin under posterior dorsal-fin anal-fin base. -

Marine Fishes of the Azores: an Annotated Checklist and Bibliography
MARINE FISHES OF THE AZORES: AN ANNOTATED CHECKLIST AND BIBLIOGRAPHY. RICARDO SERRÃO SANTOS, FILIPE MORA PORTEIRO & JOÃO PEDRO BARREIROS SANTOS, RICARDO SERRÃO, FILIPE MORA PORTEIRO & JOÃO PEDRO BARREIROS 1997. Marine fishes of the Azores: An annotated checklist and bibliography. Arquipélago. Life and Marine Sciences Supplement 1: xxiii + 242pp. Ponta Delgada. ISSN 0873-4704. ISBN 972-9340-92-7. A list of the marine fishes of the Azores is presented. The list is based on a review of the literature combined with an examination of selected specimens available from collections of Azorean fishes deposited in museums, including the collection of fish at the Department of Oceanography and Fisheries of the University of the Azores (Horta). Personal information collected over several years is also incorporated. The geographic area considered is the Economic Exclusive Zone of the Azores. The list is organised in Classes, Orders and Families according to Nelson (1994). The scientific names are, for the most part, those used in Fishes of the North-eastern Atlantic and the Mediterranean (FNAM) (Whitehead et al. 1989), and they are organised in alphabetical order within the families. Clofnam numbers (see Hureau & Monod 1979) are included for reference. Information is given if the species is not cited for the Azores in FNAM. Whenever available, vernacular names are presented, both in Portuguese (Azorean names) and in English. Synonyms, misspellings and misidentifications found in the literature in reference to the occurrence of species in the Azores are also quoted. The 460 species listed, belong to 142 families; 12 species are cited for the first time for the Azores. -

How Did the Deepwater Horizon Oil Spill Impact Deep-Sea Ecosystems? Charles R
Nova Southeastern University NSUWorks Marine & Environmental Sciences Faculty Articles Department of Marine and Environmental Sciences 9-1-2016 How Did the Deepwater Horizon Oil Spill Impact Deep-Sea Ecosystems? Charles R. Fisher Pennsylvania State University - Main Campus Paul A. Montagna Texas A & M University - Corpus Christi Tracey Sutton Nova Southeastern University, [email protected] Find out more information about Nova Southeastern University and the Halmos College of Natural Sciences and Oceanography. Follow this and additional works at: https://nsuworks.nova.edu/occ_facarticles Part of the Marine Biology Commons, and the Oceanography and Atmospheric Sciences and Meteorology Commons NSUWorks Citation Charles R. Fisher, Paul A. Montagna, and Tracey Sutton. 2016. How Did the Deepwater Horizon Oil Spill Impact Deep-Sea Ecosystems? .Oceanography , (3) : 182 -195. https://nsuworks.nova.edu/occ_facarticles/784. This Article is brought to you for free and open access by the Department of Marine and Environmental Sciences at NSUWorks. It has been accepted for inclusion in Marine & Environmental Sciences Faculty Articles by an authorized administrator of NSUWorks. For more information, please contact [email protected]. OceTHE OFFICIALa MAGAZINEn ogOF THE OCEANOGRAPHYra SOCIETYphy CITATION Fisher, C.R., P.A. Montagna, and T.T. Sutton. 2016. How did the Deepwater Horizon oil spill impact deep-sea ecosystems? Oceanography 29(3):182–195, http://dx.doi.org/10.5670/oceanog.2016.82. DOI http://dx.doi.org/10.5670/oceanog.2016.82 COPYRIGHT This article has been published in Oceanography, Volume 29, Number 3, a quarterly journal of The Oceanography Society. Copyright 2016 by The Oceanography Society. All rights reserved. USAGE Permission is granted to copy this article for use in teaching and research. -
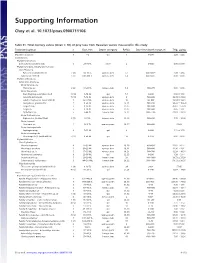
Supporting Information
Supporting Information Choy et al. 10.1073/pnas.0900711106 Table S1. Total mercury values (mean ؎ SD) of prey taxa from Hawaiian waters measured in this study Taxonomic group n Size, mm Depth category Ref(s). Day-time depth range, m THg, g/kg Mixed Zooplankton 5 1–2 epi 1 0–200 2.26 Ϯ 3.23 Invertebrates Phylum Ctenophora Ctenophores (unidentified) 3 20–30 TL other 2 0–600 0.00 Ϯ 0.00 Phylum Chordata, Subphylum Tunicata Class Thaliacea Pyrosomes (unidentified) 2 (8) 14–36 TL upmeso.dvm 2, 3 400–600ϩ 3.49 Ϯ 4.94 Salps (unidentified) 3 (7) 200–400 TL upmeso.dvm 2, 4 400–600ϩ 0.00 Ϯ 0.00 Phylum Arthropoda Subphylum Crustacea Order Amphipoda Phronima sp. 2 (6) 17–23 TL lomeso.dvm 5, 6 400–975 0.00 Ϯ 0.00 Order Decapoda Crab Megalopae (unidentified) 3 (14) 3–14 CL epi 7, 8 0–200 0.94 Ϯ 1.63 Janicella spinacauda 7 (10) 7–16 CL upmeso.dvm 9 500–600 30.39 Ϯ 23.82 Lobster Phyllosoma (unidentified) 5 42–67 CL upmeso.dvm 10 80–400 18.54 Ϯ 13.61 Oplophorus gracilirostris 5 9–20 CL upmeso.dvm 9, 11 500–650 90.23 Ϯ 103.20 Sergestes sp. 5 8–25 CL upmeso.dvm 12, 13 200–600 45.61 Ϯ 51.29 Sergia sp. 5 6–10 CL upmeso.dvm 12, 13 300–600 0.45 Ϯ 1.01 Systellapsis sp. 5 5–44 CL lomeso.dvm 9, 12 600–1100 22.63 Ϯ 38.18 Order Euphausiacea Euphausiids (unidentified) 2 (7) 5–7 CL upmeso.dvm 14, 15 400–600 7.72 Ϯ 10.92 Order Isopoda Anuropus sp. -

A Review of Lanternfishes (Families: Myctophidae and Neoscopelidae)
Zoological Studies 40(2): 103-126 (2001) A Review of Lanternfishes (Families: Myctophidae and Neoscopelidae) and Their Distributions around Taiwan and the Tungsha Islands with Notes on Seventeen New Records John Ta-Ming Wang* and Che-Tsung Chen Graduate School of Fisheries Science, National Taiwan Ocean University, Keelung, Taiwan 202, R.O.C. Fax: 886-2-28106688. E-mail: [email protected] (Accepted December 20, 2000) John Ta-Ming Wang and Che-Tsung Chen (2001) A review of lanternfishes (Families: Myctophidae and Neoscopelidae) and their distributions around Taiwan and the Tungsha Islands with notes on seventeen new records. Zoological Studies 40(2): 103-126. Lanternfishes collected during 9 cruises from 1991 to 1997 were studied. The area sampled lies between 19°N and 25°N and 114°E and 123°E. The specimens collected in this area comprise 40 species belong to 16 genera, among which 17 species are first records. These first record species include Benthosema fibulatum, Bolinichthys supralateralis, Electrona risso, Hygophum proximum, H. reinhardtii, Lampadena anomala, Lobianchia gemellarii, Lampanyctus niger, L. turneri, L. tenuiformis, Myctophum asperum, M. aurolaternatum, M. nitidulum, M. spinosum, Notolychnus valdiviae, Notoscopelus caudispinosus, and N. resplendens. Among these, six species, Bolinichthys supralateralis, Electrona risso, Lampanyctus turneri, Lampadena anomala, Notolychnus valdiviae, and Notoscopelus caudispinosus, are first records for the South China Sea, and the species, Lampadena anomala is a new record for Asian oceans (Table 1). Four species (Triphoturus microchir, Diaphus diadematus, D. latus, and D. taaningi) were controversial in previous reports, so they are discussed in this study. Geographic distributions and localities of catches of all lanternfish species are shown on the maps (Figs. -

Annotated Checklist of the Fish Species (Pisces) of La Réunion, Including a Red List of Threatened and Declining Species
Stuttgarter Beiträge zur Naturkunde A, Neue Serie 2: 1–168; Stuttgart, 30.IV.2009. 1 Annotated checklist of the fish species (Pisces) of La Réunion, including a Red List of threatened and declining species RONALD FR ICKE , THIE rr Y MULOCHAU , PA tr ICK DU R VILLE , PASCALE CHABANE T , Emm ANUEL TESSIE R & YVES LE T OU R NEU R Abstract An annotated checklist of the fish species of La Réunion (southwestern Indian Ocean) comprises a total of 984 species in 164 families (including 16 species which are not native). 65 species (plus 16 introduced) occur in fresh- water, with the Gobiidae as the largest freshwater fish family. 165 species (plus 16 introduced) live in transitional waters. In marine habitats, 965 species (plus two introduced) are found, with the Labridae, Serranidae and Gobiidae being the largest families; 56.7 % of these species live in shallow coral reefs, 33.7 % inside the fringing reef, 28.0 % in shallow rocky reefs, 16.8 % on sand bottoms, 14.0 % in deep reefs, 11.9 % on the reef flat, and 11.1 % in estuaries. 63 species are first records for Réunion. Zoogeographically, 65 % of the fish fauna have a widespread Indo-Pacific distribution, while only 2.6 % are Mascarene endemics, and 0.7 % Réunion endemics. The classification of the following species is changed in the present paper: Anguilla labiata (Peters, 1852) [pre- viously A. bengalensis labiata]; Microphis millepunctatus (Kaup, 1856) [previously M. brachyurus millepunctatus]; Epinephelus oceanicus (Lacepède, 1802) [previously E. fasciatus (non Forsskål in Niebuhr, 1775)]; Ostorhinchus fasciatus (White, 1790) [previously Apogon fasciatus]; Mulloidichthys auriflamma (Forsskål in Niebuhr, 1775) [previously Mulloidichthys vanicolensis (non Valenciennes in Cuvier & Valenciennes, 1831)]; Stegastes luteobrun- neus (Smith, 1960) [previously S. -

Trophic Structure of Midwater Fishes Over Cold Seeps in the North Central Gulf of Mexico
TROPHIC STRUCTURE OF MIDWATER FISHES OVER COLD SEEPS IN THE NORTH CENTRAL GULF OF MEXICO Jennifer P. McClain-Counts A Thesis Submitted to the University of North Carolina Wilmington in Partial Fulfillment of the Requirements for the Degree of Master of Science Center for Marine Science University of North Carolina Wilmington 2010 Approved by Advisory Committee Steve W. Ross Lawrence B. Cahoon Chair Joan W. Willey Accepted by Dean, Graduate School TABLE OF CONTENTS ABSTRACT....................................................................................................................... iv ACKNOWLEDGMENTS ................................................................................................. vi DEDICATION.................................................................................................................. vii LIST OF TABLES........................................................................................................... viii LIST OF FIGURES ........................................................................................................... xi INTRODUCTION ...............................................................................................................1 METHODS ..........................................................................................................................4 Study Area................................................................................................................4 Sample Collection ....................................................................................................5 -

"Especies, Ensamblajes Y Paisajes De Los Bloques
“ESPECIES, ENSAMBLAJES Y PAISAJES DE LOS BLOQUES MARINOS SUJETOS A EXPLORACIÓN DE HIDROCARBUROS” CARACTERIZACIÓN DE LA MEGAFAUNA Y EL PLANCTON DEL PACÍFICO COLOMBIANO Convenio específico de cooperación No. 008 de 2008 INFORME DE ACTIVIDADES INVEMAR – ANH Fase III - Pacífico Vinculado al Ministerio de Ambiente, Vivienda y Desarrollo Territorial Santa Marta, Octubre 31 de 2010 “ESPECIES, ENSAMBLAJES Y PAISAJES DE LOS BLOQUES MARINOS SUJETOS A EXPLORACIÓN DE HIDROCARBUROS” CARACTERIZACIÓN DE LA MEGAFAUNA Y EL PLANCTON DEL PACÍFICO COLOMBIANO INFORME DE ACTIVIDADES Directivos INVEMAR Coordinación INVEMAR Francisco A. Arias Isaza, M. Sc, Dr. Director General David A. Alonso Carvajal, M. Sc. Francisco Armando Arias Isaza Milena Benavides Serrato., M. Sc. Subdir ector Coordinación ANH Coordinación de Investigaciones Boris Navarro Jesús Antonio Garay Tinoco GRUPO DE INVESTIGACIÓN Subdirector Componente de biodiversidad Recursos y Apoyo a la Investigación Carlos Augusto Pinilla González Adriana Gracia, M. Sc. Biología Marina Andrea Polanco, M. Sc. Biología Marina Coordinador Programa Andrés Merchán, M. Sc. Biología Marina Biodiversidad y Ecosistemas Marinos Christian Díaz, B. Sc. Biología Marina David A. Alonso Carvajal Erika Montoya C., B. Sc. Biología Marina Erlenis Fontalvo, B. Sc. Biología Johanna Medellín, B. Sc. B iología Marina Coordinadora Programa Martha Díaz Ruíz, M. Sc. Biología Marina Investigación para la Gestión Marina y Milena Benavides, M. Sc. Biología Marina Costera Manuel Garrido Linares, B. Sc. Biología Paula Cristina Sierra Correa Paola Flórez, B. Sc. Biología Marina Componente análisis áreas significativas Luis A. Mejía, B. Sc. Biología Marina para la biodiversidad Ana M. Lagos, Estudiante. Biología Coordinadora Programa Vanesa Izquierdo, Estudiante Biología David A. Alonso Carvajal, M. Sc.