A Test Using Drosophila Melanogaster
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
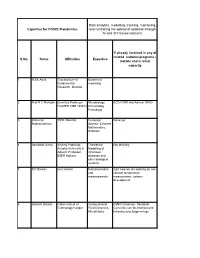
Data Analytics and Modeling
Data analytics, modelling, tracking, monitoring, Expertise for COVID /Pandemics and controlling the spread of outbreak through AI and IOT-based solutions If already involved in any of related national programs : S.No. Name Affiliation Expertise Details and in what capacity 1 H. M. Antia Tata Institute of Numerical Fundamental modelling Research, Mumbai 2 Prof R C Mahajan Emeritus Professor Microbiology ECD ICMR and Advisor WHO PGIMER CHD 160012 Immunology Parasitogy 3 Jaikumar TIFR, Mumbai Computer None yet. Radhakrishnan Science, Discrete Mathematics, Software 4 Somdatta Sinha Visiting Professor, Theoretical Not officially. Ashoka University & Modelling of Adjunct Professor, infectious IISER Kolkata diseases and other biological systems 5 S K Biswas iiser mohali Instrumentation right now we are working on non and contact temperature measurements measurement system development 6 Gautam Biswas Indian Intitute of Computational GIAN (Chairman, Sectional Technology Kanpur Fluid Dynamics, Committee on Mechanical and Microfluidics Infrastructure Engineering) 7 Ram Ramaswamy IIT Delhi Modeling - 8 Sandip Paul Ramanujan Fellow NGS data NA analysis, adaptive evolution 9 Amish kumar Ph.D. Student Bioinformatics After submission of my Ph.D thesis I am working as visitor research student at Department of plant sciences under Project entitled as "TIGR2ESS: Transforming India's Green Revolution by Research and Empowerment for Sustainable food Supplies". 10 Surajit Assistant Professor, Immunology, None Bhattacharjee Department of Infection Biology Molecular -

Year : 2015-16
LIST OF RTI Nodal Officers Year : 2015-16 Sr.No Ministry/Department/Organisation Nodal Officer Contact Address Phone No/Fax/ Email Name/Designation 1 Cabinet Secretariat Shri Sunil Mishra Rashtrapati Bhawan Director & CPIO New Delhi 1 Cabinet Secretariat 110001 23018467 2 Department of Atomic Energy Shri P Ramakrishnan Central Office, Western Chief Administrator Anushaktinagar, 1 Atomic Energy Education Society Mumbai - 400 094 02225565049 [email protected] Dr. Pankaj Tandon Niyamak Bhavan, Scientific Officer F Anushaktinagar, 2 Atomic Energy Regulatory Board Mumbai- 400 094 25990659 [email protected] Shri M.B. Verma Atomic Minerals Atomic Minerals Directorate for Additional Director (Op 1-10-153/156, AMD 3 Exploration and Research Hyderabad - 500 016 04027766472 [email protected] Dr. P.M. Satya Sai Head, Commissioning 04427480044 Scientific Officer (H) Nuclear Recycle Group 4427480252 4 BARC Facilities, Kalpakkam Kalpakkam, [email protected] Shri Sanjay Pradhan TNRPO, NRB, BARC, 02525244299 Chief Superitendent Ghivali Post, Palghar 2525244158 5 Bhabha Atomic Resarch Centre (Tarapur) Pin 401502 [email protected] Shri. B. P. Joshi Central Complex, 3rd 02225505330 Chief Administrative Trombay, 2225505151 6 Bhabha Atomic Research Centre Mumbai - 400 085 [email protected] Smt. K. Malathi BHAVINI 04427480915 Bharatiya Nabhikiya Vidhyut Nigam Ltd. Senior Manager (HR) Kalpakkam - 603 102 4427480640 7 (BHAVINI) Kancheepuram Dist. [email protected] Shri M.C. Dinakaran BRIT, Project House, 02225573534 Board of Radiation and Isotope General Manager (PIO) Anushakti Nagar 2225562161 8 Technology Mumbai- 400 094. [email protected] Deputy Secretary Anushakti Bhavan 22839961 Deputy Secretary CSM Marg, 22048476 9 Department of Atomic Energy Mumbai-400001 [email protected] Shri V.K. -

Basic Research : Its Role In
Published by: The National Academy of Sciences, India (NASI) Year of Publication: 2018 For further information, please contact: The National Academy of Sciences, India (NASI) 5, Lajpat Rai Road, Allahabad, Uttar Pradesh-211002 Phone: +91 (0532) 2640224, Fax: +91 (532) 2641183 Email: [email protected]; [email protected] Website: www.nasi.ac.in PROCEEDINGS OF THE SYMPOSIUM ON BASIC RESEARCH – IT’S ROLE IN NATIONAL DEVELOPMENT 87TH ANNUAL SESSION OF NASI 8TH -10TH DEC 2017 Venue:- Savitribai Phule Pune University, Ganeshkhind Road, Pune CONTENTS FOREWORD PREFACE I. INAUGURAL SESSION Welcome Address 3 Prof. Nitin R. Karmalkar, Vice-Chancellor, Savitribai Phule Pune University About the Pune Chapter 5 Dr. Dilip Dattaray Dhavale, Chairman, NASI Local Chapter About the Annual Symposium 7 Dr. Manju Sharma (Convener) Distinguished Woman Scientist Chair, NASI and Former Secretary, Department of Biotechnology, Government of India Role of Space Research in National Development 9 Dr. Kiran Kumar (Guest of Honour) Secretary, Department of Space, Chairman, Space Commission and Chairman, ISRO Presidential Address 18 Dr. Anil Kakodkar, President, NASI Inaugural Address 20 Shri C. H. Vidyasagar Rao, Governor of Maharashtra Vote of Thanks 24 Prof. Veena Tandon, General Secretary, NASI Game Changing in Indian Science, Technology and Innovation 26 Dr. R. A. Mashelkar, FRS (Chief Guest) National Research Professor Theme Publication: Basic Research as an Integral Component of a Self Reliant 33 Base of Science and Technology (Its Role, Relevance, Support, Areas of Thrust) Late Prof. M.G.K. Menon, FRS Invited Article: Basic Science: A Clinician’s Perception 64 Prof. P.N. Tandon, National Research Professor and Former President, NASI II. -

Contemporary Excitement in New Biology NAGALAND UNIVERSITY
UGC Sponsored National Conference on Contemporary Excitement in New Biology National Advisors (CENB- 2018) Prof. SN. Hegde, Mysuru October 30-31, 2018 Prof. M. Venkateswarlu, Shimoga Prof. LS. Shashidhara, Pune Organized by Prof. Amitabh Joshi, Bangalore Dr. T. Madhan Mohan, New Delhi Dr. Kumarasamy Thangaraj Hyderabad Prof. Pawan Sharma, New Delhi Prof. Jagat Kumar Roy, Varanasi Prof. RNS. Yadav, Dibrugarh NAGALAND UNIVERSITY Prof. Ramesh Sharma, Shillong Prof. S. Rama Rao, Shillong (A Central University) Prof. Prabodh Bora, Guwahati Prof. Prasad Burra, Haryana DEPARTMENT OF ZOOLOGY Prof. DK. Sharma, Guwahati Headquarters: LUMAMI, ZUNHEBOTO DISTRICT- Dr. Longvah, Hyderabad 798627 NAGALAND, INDIA Prof. Neelu Nangia, Bangalore Prof. Kamal Jaiswal, Lucknow Prof. Kaushal, Nainital Organizing Committee Prof. OP. Singh, Shillong Prof. BBP. Gupta, Shillong Patron Prof. SB. Prasad, Shillong Prof. Pardeshi Lal Vice Chancellor, Nagaland University Convener(s) Prof. SU. Ahmed Prof. LN. Kakati Department of Zoology, Nagaland Confirmed Speakers: University Prof. Anupam Chatterjee (NEHU, Shillong, Organizing Secretary Meghalaya (Keynote speaker) Prof. Sarat Chandra Yenisetti Prof. BK. Konwar (Tejpur University, Head, Department of Zoology, Nagaland Assam) University Prof. KRS. Sambasiva Rao (Mizoram University, Mizoram) Treasurer Prof. JC. Kalita (Guwahati University, Dr. Bendang Ao Assam) Department of Zoology, Nagaland Prof. Jharna Chakraborty (Rajiv Gandhi University University, Arunachal Pradesh) Prof. AK. Mukherjee (Tejpur University, Members -

Indian National Science Academy Bahadur Shah Zafar Marg, New Delhi - 110002
INDIAN NATIONAL SCIENCE ACADEMY BAHADUR SHAH ZAFAR MARG, NEW DELHI - 110002 Minutes of the General Body Meeting of the Indian National Science Academy held on 27 July, 2017 in the Academy premises. The following Fellows were present: Professor Ajay K Sood, President, INSA Professor Anurag Sharma, Vice-President (Fellowship Affairs) Professor Kankan Bhattacharyya, Vice-President (Science Promotion) Professor NR Jagannathan, Vice-President (Resource Management) Professor SC Lakhotia, Vice-President (Informatics and Publications) Professor LS Shashidhara, Vice-President (Science & Society) Professor Faizan Ahmad Professor Talat Ahmad Dr AC Banerjea Professor Subhasis Chaudhuri Dr Madhu Dikshit Dr Kunal Ghosh Dr Swapan K Ghosh Professor LC Gupta Professor Amitabh Joshi Dr Sushil Kumar Professor Nibir Mandal Professor NK Mehra Professor HY Mohan Ram Dr Ganesh Pandey Professor G Parthasarathy Professor IBS Passi Professor Sarva Jit Singh Professor Somdatta Sinha Professor PN Tandon Dr KC Upadhyaya Professor K Veluthambi President, INSA welcomed all the Fellows to the General body meeting. Thereafter, the regular agenda items were taken up. 1. Condolence at the passing away of the following distinguished Fellows: The sad demise of Professors Bhola Nath Dhawan, Arumugam Gnanam, Udupi Ramachandra Rao, Arun Kumar Sharma and Yash Pal distinguished Fellows of the Academy was reported. The obituary notes were read by the President and all those present stood in silence for a minute as a mark of respect to the deceased. 2. Confirmation of minutes of Ordinary General Meeting held on 26 April, 2017. The minutes of the Ordinary General Meeting held on 26 April, 2017 were read by Professor Anurag Sharma, Vice-President. -

Contemporary Issues in Evolutionary Biology
Journal of Genetics, Vol. 96, No. 3, July 2017, pp. 399–400 DOI 10.1007/s12041-017-0806-7 Contemporary issues in evolutionary biology Preface We are delighted to bring to the readers, a set of peer-reviewed papers on evolutionary biology, published as a special issue of the Journal of Genetics. These papers emanated from ruminations upon and discussions at the Foundations of Evolutionary Theory: the Ongoing Synthesis meeting at Coorg, India, in February 2014, and the Foundations of Biology meetings at Pune, India, in April 2015 and March 2016. One of us (LSS), along with Sutirth Dey (IISER Pune) and Amitabh Joshi (JNCASR, Bengaluru), organized these meetings in order to bring together a diverse set of interested academics to discuss and argue about fundamental issues in the subject. These discussions included, among others, the possible consequences of nonDNA-based inheritance—epigenetics and cultural evolution, niche construction, and developmental mechanisms on our understanding of the evolutionary process, speciation, complexity in biology, and constructing a formal evolutionary theory. This special issue begins with an original article by K. P. Mohanan on the Conceptual Foundations of Evolution- ary Thought, in which evolution is conceptualized as symmetry breaking, thereby attempting to integrate physical, biological (macroevolution), and cultural (societal) evolution. Biological evolution and cultural evolution are fur- ther examined conceptually as the emergence and persistence of traits rather than taxa. Structural and functional constraints are invoked to explain the contrast between the emergence of novelty and the resistance to novelty (per- sistence). The second article presents a review of various species concepts, and thoughts about species concepts versus the delimitation of species. -

Jawharalal Nehru Annual Rep-2011-12
ISSN.0973-9319 ANNUAL REPORT 2011-2012 JAWAHARLAL NEHRU CENTRE FOR ADVANCED SCIENTIFIC RESEARCH (A Deemed to be University) Jakkur, Bangalore – 560 064. Website: http://www.jncasr.ac.in CONTENTS Page No. The Centre Foreword ........................................................................................................................................... 1 Introduction .......................................................................................................................................... 3 Objectives ........................................................................................................................................... 4 Progress ........................................................................................................................................... 5 Highlights of research and other activities....................................................................................... 7 Activities Chart ..................................................................................................................................... 12 Organisation Chart .............................................................................................................................. 13 The Organisation Council of Management ...................................................................................................................... 14 Finance Committee .............................................................................................................................. -

Female Asian Elephants in Nagarahole National Park, Southern India ” Has Been Carried out by Mr
RESOURCE AVAILABILITY , WITHIN -CLAN AND BETWEEN -CLAN AGONISTIC INTERACTIONS , AND DOMINANCE RELATIONSHIPS AMONGST FEMALE ASIAN ELEPHANTS IN NAGARAHOLE NATIONAL PARK , SOUTHERN INDIA A thesis submitted for the degree of Doctor of Philosophy by Hansraj Gautam Evolutionary and Organismal Biology Unit Jawaharlal Nehru Centre for Advanced Scientific Research, Bengaluru 560064, India. September 2019 ii CERTIFICATE This is to certify that the work presented in this thesis titled “ Resource Availability, Within-Clan and Between-Clan Agonistic Interactions, and Dominance Relationships amongst Female Asian Elephants in Nagarahole National Park, Southern India ” has been carried out by Mr. Hansraj Gautam under my supervision at the Evolutionary and Organismal Biology Unit, Jawaharlal Nehru Centre for Advanced Scientific Research, Bengaluru, India, and that the results in this thesis have not previously formed the basis for the award of any other degree, diploma, or fellowship. Date: Prof. T.N.C. Vidya iii iv DECLARATION I declare that the matter presented in my thesis titled “Resource Availability, Within-Clan and Between-Clan Agonistic Interactions, and Dominance Relationships amongst Female Asian Elephants in Nagarahole National Park, Southern India ” is the result of studies carried out by me at the Evolutionary and Organismal Biology Unit of the Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore, India, under the supervision of Prof. T.N.C. Vidya, and that this work has not been submitted elsewhere for any other degree. In keeping with the general practice of reporting scientific observations, due acknowledgement has been made wherever the work described has been based on the findings of other investigators. Any omission, which might have occurred by oversight, is regretted. -

Activity Report (2013-2015)
2 0 1 3 ICTS— 2 0 1 5 REPORTTATA INSTITUTE OF FUNDAMENTAL RESEARCH International Centre for Theoretical Sciences (ICTS-TIFR) Shivakote, Hesaraghatta Hobli, Bengaluru North 560 089 www.icts.res.in ii ICTS REPORT 2013-15 | DIRECTOR’S REPORT ICTS REPORT 2013-15 | DIRECTOR’S REPORT iii CONTENTS 1) DIRECTOR’S REPORT PAGE 1 2) RESEARCH REPORTS PAGE 7 3) ASSOCIATE FACULTY PAGE 69 4) ACADEMIC ACTIVITIES PAGE 77 5) OUTREACH PAGE 163 6) CAMPUS PAGE 173 7) STAFF PAGE 179 8) ORGANIZATION PAGE 182 9) AWARDS AND HONORS PAGE 184 Editor: Ananya Dasgupta Design: Anisha Heble Photographs: Anbujawahar iv ICTS REPORT 2013-15 | DIRECTOR’S REPORT ICTS REPORT 2013-15 | DIRECTOR’S REPORT 1 DIRECTOR’S REPORT 2 ICTS REPORT 2013-15 | DIRECTOR’S REPORT ICTS REPORT 2013-15 | DIRECTOR’S REPORT 3 Several members of our faculty have been recognized with awards, grants and fellowships within India as well as internationally. The details have been provided within the report. ICTS was awarded in 2015 the prestigious ‘Targeted Grants to Institutes’ from the Simons Foundation, USA. The AIRBUS Corporate Foundation grant for an international teaching and research chair entitled ‘Mathematics of Complex Systems’, which ICTS shares with the Centre for Applicable Mathematics, underwent a successful annual review and continues. Both these grants lend enormous encouragement to a fledgling Centre and enhance the ability of ICTS to conduct programs, invite outstanding post- docs and long term visiting professors. The new ICTS Campus has started functioning. The campus is equipped with a modern library that will provide access to books and electronic resources on advanced topics; state-of-the-art computing and networking infrastructure; a data centre with high-speed connectivity and extensive storage facility for doing big-data sciences; conference and lecture halls equipped with high-end audio-video equipment for recording and broadcasting ICTS programs; healthcare, childcare and recreational facilities for members and visitors. -

Resonance Journal of Science Education
Resonance journal of science education Editorial Board Chief Editor S Mahadevan Associate Editors J H Arakeri, Bangalore D N Rao, Bangalore G Nagendrappa, Bangalore Renuka Ravindran, Bangalore Vasant Natarajan, Bangalore K L Sebastian, Bangalore Biman Nath, Bangalore Priti Shankar, Bangalore D P Patil, Bangalore Council of Editors K B Athreya, USA Bikram Phookun, New Delhi Anand K Bachhawat, Chandigarh C R Pranesachar, Bangalore V Balakrishnan, Chennai T P Radhakrishnan, Hyderabad B V Rajaram Bhat, Bangalore V Rajaraman, Bangalore Dipankar Bhattacharya, Bangalore S Ramasubramanian, Bangalore M K Chandrashekaran, Bangalore K Sankara Rao, Bangalore S R Gadre, Pune D M Salunke, New Delhi S R Ghorpade, Mumbai L S Sashidhara, Hyderabad A P Gore, Pune Shailesh A Shirali, Chickmagalur Amitabh Joshi, Bangalore Mewa Singh, Mysore · G Marimuthu, Madurai J Srinivasan, Bangalore N Mukunda, Bangalore Akhilesh K Tyagi, New Delhi Utpal Nath, Bangalore Raghavan Varadarajan, Bangalore Editor of Publications N Mukunda, Indian Institute of Science, Bangalore Assistant Editors M Raj Lakshmi, Sujatha Mohankumar Production G Chandramohan Editorial Staff G Madhavan, R Pushpa, M Srimathi, Circulation and Accounts Shanthi Bhasker, B V Bhoja Raja, Peter Jayaraj, B Krishna, Ranjini Mohan, C Vedamurthy, C Visweswaraiah Annual Subscription Rates Institutions: Rs.400/-; Individuals: Rs.350/- minimum for two years; Price per single copy: Rs.40/- All correspondence regarding subscription should be addressed to The Circulation Department of the Academy. Email:[email protected]; Online orders for India are also accepted at Scholars without Borders (www.scholarswithoutborders.in) Email:[email protected] Editorial Office: Indian Academy of Sciences, C V Raman Avenue, PB No. 8005, Bangalore 560 080, India. -

Academic Events 2008-2009
Vision Create research Universities of the highest caliber in which teaching and education will be totally integrated with the state of the art research. Evolve undergraduate and post-graduate teaching in an intellectually vibrant atmosphere of research. Make education and careers in basic sciences more exciting through excellence in integrative teaching and learning of sciences Mission Create quality education and research in basic sciences that will nurture curiosity and creativity Combine undergraduate and postgraduate teaching with cutting edge research in frontier areas. Provide entry into research at a younger age through flexible borderless curriculum and research projects Forge actively strong relationships with existing universities and colleges and network with laboratories and institutions Establish advanced research laboratories and central facilities to encourage interdisciplinary and Our Values Dedicate to learn, teach and serve the society through excellence in education, research and public service Celebrate the spirit of academic excellence Create learning and working environment based on integrity, fairness, dignity and professionalism to provide equal opportunities for everyone Encourage creativity of students, faculty and staff, recognising individual and collaborative achievements Strive to develop tolerance of diversity in community, free of all acts of discrimination and harassment Respect, support and value the civil and respectful expression of individual beliefs and opinions Develop and encourage sense of environmental responsibility KALPA 2009 where tomorrow's science begins today Campus - I : 900, NCL Innovation Park, Dr. Homi Bhabha Road, Pune 411 008. Tel.: 020 - 2589 8021 / 23 Campus - II : Sai Trinity Building, Garware Circle, Pashan, Pune 411 021. Tel.: 020 - 2590 8000 www.iiserpune.ac.in The Chairman & Members of Board of Governors, IISER, Pune 1. -
Annual Report 2018-19 Annual Report 02 Admissions and Financial Aid
ANNUAL REPORT Ahmedabad University To foster continuous progress of self and society 2018 - 2019 CONTENTS Year at a Glance 02 • Vice Chancellor's Message 04 Schools • Amrut Mody School of Management 06 • School of Arts and Sciences 20 • School of Engineering and Applied Science 38 • School of Computer Studies 49 Centres • Centre for Heritage Management 53 • Global Centre for Environment and Energy 58 • VentureStudio 62 • Physiotherapy College for the Visually Impaired 67 • Centre for Learning Futures 68 Career Development Centre 72 University Research Board 76 Life at Ahmedabad University • Key Events at the University 84 • SSETU: Wellness for All 88 Faculty and Staff 93 Financial Statement 98 University Governance • Board of Governors 110 • Board of Management 111 • Advisory and other Committees 112 Ahmedabad University is a liberal arts driven environment with a difference and be at the frontiers research university. It is dedicated to rigorous of cutting-edge global research across the natural, academic pursuit with a focus on building inquiry physical and social sciences, humanities, and as a value through interdisciplinary learning. We performing and visual arts. It offers interdisciplinary prepare students to think deeply and creatively programmes at undergraduate and graduate across fields, and emerge as independent thinkers levels. The School of Engineering and Applied and compassionate leaders who can innovatively Science is one of the most unique engineering engage with the complex challenges of our society. schools in India with an engineering foundation Our research and teaching spans humanities and spanning several disciplines (based on the premise languages, social sciences, mathematical and that all engineers must know many disciplines to physical sciences, biological and life sciences, be able to solve complex problems) and a unique performing and visual arts, engineering, and hands-on experiential learning environment (i.e., management.