The Perception of Color from Motion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geometry of Some Functional Architectures of Vision
Singular Landscapes: in honor of Bernard Teissier 22-26 June, 2015 Geometry of some functional architectures of vision Jean Petitot CAMS, EHESS, Paris J. Petitot Neurogeometry Bernard and visual neuroscience Bernard helped greatly the developement of geometrical models in visual neuroscience. In 1991 he organized the first seminars on these topics at the ENS and founded in 1999 with Giuseppe Longo the seminar Geometry and Cognition. From 1993 on, he organized at the Treilles Foundation many workshops with specialists such as Jean-Michel Morel, David Mumford, G´erard Toulouse, St´ephaneMallat, Yves Fr´egnac, Jean Lorenceau, Olivier Faugeras. He organised also in 1998 with J.-M. Morel and D. Mumford a special quarter Mathematical Questions on Signal and Image processing at the IHP. He worked with Alain Berthoz at the College de France (Daniel Bennequin worked also a lot there on geometrical models in visual neuroscience). J. Petitot Neurogeometry Introduction to Neurogeometry In this talk I would try to explain some aspects of Neurogeometry, concerning the link between natural low level vision of mammals and geometrical concepts such as fibrations, singularities, contact structure, polarized Heisenberg group, sub-Riemannian geometry, noncommutative harmonic analysis, etc. I will introduce some very basic and elementary experimental facts and theoretical concepts. QUESTION: How the visual brain can be a neural geometric engine? J. Petitot Neurogeometry The visual brain Here is an image of the human brain. It shows the neural pathways from the retina to the lateral geniculate nucleus (thalamic relay) and then to the occipital primary visual cortex (area V 1). J. Petitot Neurogeometry fMRI of human V1 fMRI of the retinotopic projection of a visual hemifield on the corresponding V1 (human) hemisphere. -

Important Processes Illustrated September 22, 2021
Important Processes Illustrated September 22, 2021 Copyright © 2012-2021 John Franklin Moore. All rights reserved. Contents Introduction ................................................................................................................................... 5 Consciousness>Sense .................................................................................................................... 6 space and senses ....................................................................................................................... 6 Consciousness>Sense>Hearing>Music ..................................................................................... 18 tone in music ........................................................................................................................... 18 Consciousness>Sense>Touch>Physiology ................................................................................ 23 haptic touch ............................................................................................................................ 23 Consciousness>Sense>Vision>Physiology>Depth Perception ................................................ 25 distance ratio .......................................................................................................................... 25 Consciousness>Sense>Vision>Physiology>Depth Perception ................................................ 31 triangulation by eye .............................................................................................................. -

Subject Index
Subject Index Absence, 47, 79, 83, 87, 89, 131, 143, 204, Appearances, 8, 10, 11, 17, 19, 51, 96, 112, 227, 230, 232, 265, 267, 268, 271, 272, 279, 121, 123, 127, 128, 130, 159, 162, 180, 315, 317, 318, 320–325, 354, 373 184–192, 224, 242, 261, 288, 289, 292, Abstraction, 6, 172, 174, 176, 178, 187, 194, 322–325, 335, 350–352, 356, 357, 359, 361, 338, 354, 368, 369, 373, 375, 400, 402, 404, 365, 373, 406 412 Art, 17, 20, 31, 93, 131, 338, 399–404, 411– Accident, 128, 144, 145, 189, 204, 212, 213, 420 234, 360, 364 abstract art, 20, 401–405, 411, 412 Accommodation, 203, 211, 224–226 color, 402, 403–404 Action-perception synergies, 131, 132, 133 compositional techniques, 399–404 Activation zone, 375 constructivism, 396, 398, 406, 407, 412, Active vision, 131 418 Adaptive strategies, 85, 96, 97 generative art, 20, 411, 412, 414–418 Additive scanning, 364 history, 184, 393 Aesthetics, 2, 9, 16, 17, 85, 90, 91, 93, 97, 113, modulation, 83, 402–403 121, 413 Pop/Op, 31, 113, 124, 352 Affine distortion, 220, 223 Artificial intelligence, 1, 28, 31 Affordance, 14, 15, 186, 338, 356, 359 Artistic expression, 83, 85, 90, 91, 97 Afterimage, 112, 149, 150 Astigmatism, 112 Alphabet, 271, 347, 351, 367 Attention, 6, 51, 84, 96, 99, 100, 125, 174, Amodal, 47, 315, 336 190, 355, 356, 375, 376, 394, 396, 397 boundaries, 263, 266 Attneave points, 5, 125, 129 completion, 175, 261, 265, 270, 273, 315, Auditory objects, 340 318, 322–324, 328, 329, 374, 376 Auffälligkeit, 375 contours, 8, 15, 19, 263, 280 Automatic processes, 4, 32, 85, 96, 97, 100, perception, -
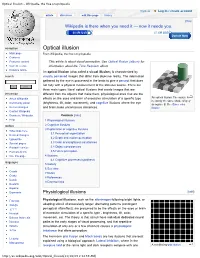
Optical Illusion - Wikipedia, the Free Encyclopedia
Optical illusion - Wikipedia, the free encyclopedia Try Beta Log in / create account article discussion edit this page history [Hide] Wikipedia is there when you need it — now it needs you. $0.6M USD $7.5M USD Donate Now navigation Optical illusion Main page From Wikipedia, the free encyclopedia Contents Featured content This article is about visual perception. See Optical Illusion (album) for Current events information about the Time Requiem album. Random article An optical illusion (also called a visual illusion) is characterized by search visually perceived images that differ from objective reality. The information gathered by the eye is processed in the brain to give a percept that does not tally with a physical measurement of the stimulus source. There are three main types: literal optical illusions that create images that are interaction different from the objects that make them, physiological ones that are the An optical illusion. The square A About Wikipedia effects on the eyes and brain of excessive stimulation of a specific type is exactly the same shade of grey Community portal (brightness, tilt, color, movement), and cognitive illusions where the eye as square B. See Same color Recent changes and brain make unconscious inferences. illusion Contact Wikipedia Donate to Wikipedia Contents [hide] Help 1 Physiological illusions toolbox 2 Cognitive illusions 3 Explanation of cognitive illusions What links here 3.1 Perceptual organization Related changes 3.2 Depth and motion perception Upload file Special pages 3.3 Color and brightness -

Named Optical Illusions
Dr. John Andraos, http://www.careerchem.com/NAMED/Optical-Illusions.pdf 1 Named Optical Illusions © Dr. John Andraos, 2003 - 2011 Department of Chemistry, York University 4700 Keele Street, Toronto, ONTARIO M3J 1P3, CANADA For suggestions, corrections, additional information, and comments please send e-mails to [email protected] http://www.chem.yorku.ca/NAMED/ Ames window Ames, A. Jr. Psychological Monographs , 1951 , 65 , No. 324 Pastore, N. Psychological Rev. 1952 , 59 , 319 Benham's top Benham, C.E. Nature 1895 , 51 , 321 von Campenhausen, C.; Schramme, J. Perception 1995 , 24 , 695 Benussi's figure (stereokinetic effect) (1927) 2 Bezold effect von Bezold, W. Die Farbenlehre im hinblick auf kunst undkunstgewerbe , Braunschweig: Berlin, 1862 Binocular vision (stereopsis) Wheatstone, C. Phil. Trans. Roy. Soc. 1838 , 128 , 371 Wheatstone, C. Phil. Trans. Roy. Soc. 1852 , 142 , 1 Café wall illusion Gregory, R.L.; Heard, P. Perception , 1979 , 8, 365 Dr. John Andraos, http://www.careerchem.com/NAMED/Optical-Illusions.pdf 3 Craik-O'Brien-Cornsweet illusion (effect) O'Brien, V. J. Opt. Soc. Am. 1958 , 48 , 112 Craik, K.J.W. The Nature of Psychology: a selection of papers essays and other writings , Cambridge University Press: Cambridge, MA, 1966 Cornsweet, T.N. Visual Perception, Academic Press: New York, 1970 Delboeuf illusion Delboeuf, J.L.R. Bull. Acad. Roy. Belg. 1892 , 24 , 545 Duchamp's figure (rotoreliefs) (1935) Ebbinghaus (or Titchener) illusion 4 Ebbinghaus, H. Z. Psychol. 1897 , 13 , 401 Titchener, E.B. Lectures on the Elementary Psychology of Feeling and Attention , Macmillan: New York, 1908 Ehrenstein illusion (figure) Ehrenstein, W. Z. Psychol. 1941 , 150 , 83 Emmert's law Emmert, E. -

ALAN GOVE,T STEPHEN GROSSBERG,2 AND
Visual Neuroscience(1995), 12, 1027-1052. Printed in the USA. Copyright @ 1995 Cambridge University Press0952-5238/95 $11.00 + .10 ALAN GOVE,t STEPHEN GROSSBERG,2 AND ENNIO MINGOLLA2 IM1T Lincoln Laboratory, 244 Wood Street, Lexington 20epartment of Cognitive and Neural Systemsand Center for Adaptive Systems, Boston University, Boston (RECEIVED November 30, 1994; ACCEPTED April 4, 1995) Abstract A neural network model is developed to explain how visual thalamocortical interactions give rise to boundary percepts such as illusory contours and surface percepts such as filled-in brightnesses.Top-down feedback interactions are needed in addition to bottom-up feed-forward interactions to simulate these data. One feedback loop is modeled between lateral geniculatenucleus (LGN) and cortical area VI, and another within cortical areas VI and V2. The first feedback loop realizesa matching process which enhances LGN cell activities that are consistent with those of active cortical cells, and suppressesLGN activities that are not. This corticogeniculate feedback, being endstoppedand oriented, also enhances LGN ON cell activations at the ends of thin dark lines, thereby leading to enhanced cortical brightness percepts when the lines group into closed illusory contours. The second feedback loop generatesboundary representations, including illusory contours, that coherently bind distributed cortical features together. Brightness percepts form within the surface representationsthrough a diffusive filling-in processthat is contained by resistive gating -

The Watercolor Illusion and Neon Color Spreading: a Unified Analysis of New Cases and Neural Mechanisms
B. Pinna and S. Grossberg Vol. 22, No. 10/October 2005/J. Opt. Soc. Am. A 1 The watercolor illusion and neon color spreading: a unified analysis of new cases and neural mechanisms Baingio Pinna Facoltà di Lingue e Letterature Straniere, Università di Sassari, via Roma 151, I-07100, Sassari, Italy Stephen Grossberg Department of Cognitive and Neural Systems and Center for Adaptive Systems, Boston University, 677 Beacon Street, Boston, Massachusetts 02215 Received January 18, 2005; accepted April 14, 2005 Coloration and figural properties of neon color spreading and the watercolor illusion are studied using phe- nomenal and psychophysical observations. Coloration properties of both effects can be reduced to a common limiting condition, a nearby color transition called the two-dot limiting case, which clarifies their perceptual similarities and dissimilarities. The results are explained by the FACADE neural model of biological vision. The model proposes how local properties of color transitions activate spatial competition among nearby per- ceptual boundaries, with boundaries of lower-contrast edges weakened by competition more than boundaries of higher-contrast edges. This asymmetry induces spreading of more color across these boundaries than con- versely. The model also predicts how depth and figure–ground effects are generated in these illusions. © 2005 Optical Society of America OCIS codes: 330.0330, 330.1720, 330.4060, 330.4270, 330.5020, 330.5510, 330.7310. 1. INTRODUCTION: A CURRENT VIEW OF One timely piece of evidence was the Thorell et al.1 study, A SEMINAL DISCOVERY OF DEVALOIS which supported the early prediction that these bound- aries and surfaces are processed by the interblob and blob Russell DeValois and his colleagues discovered many streams, respectively, from V1 to V4. -
Chapter Four the Mysterious Universe of James Jeans
Durham E-Theses Popular Theology from Popular Scientists: Assessing the Legacy of Eddington and Jeans as Apologists REYNOLDS, JONATHAN,OWEN How to cite: REYNOLDS, JONATHAN,OWEN (2017) Popular Theology from Popular Scientists: Assessing the Legacy of Eddington and Jeans as Apologists , Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/12370/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk 2 Popular Theology from Popular Scientists: Assessing the Legacy of Eddington and Jeans as Apologists Abstract This thesis asserts and demonstrates that the current historical evaluation of the significance of Arthur Eddington and James Jeans is inadequate. Not only has their importance in the years between the two World Wars been forgotten, but their transitional role in the science and religion debate post-Darwin is now largely unrecognised. Both had a major influence on subsequent popular apologists and Eddington in particular influenced post war academic theologians as diverse as Thomas Torrance and Eric Mascall. -
Zur Erlangung Des Grades Eines Doktors Der Naturwissenschaften
Adaptations of the human eye to reduce the impact of chromatic aberrations on vision Dissertation zur Erlangung des Grades eines Doktors der Naturwissenschaften der Mathematisch-Naturwissenschaftlichen Fakultät und der Medizinischen Fakultät der Eberhard-Karls-Universität Tübingen vorgelegt von Yun Chen aus Shanghai, China July – 2015 Tag der mündlichen Prüfung: 30,Oct,2015 Dekan der Math.-Nat. Fakultät: Prof. Dr. W. Rosenstiel Dekan der Medizinischen Fakultät: Prof. Dr. I. B. Autenrieth 1. Berichterstatter: Prof. Dr. Frank Schaeffel 2. Berichterstatter: Prof. Dr. Uwe IIg Prüfungskommission: Prof. Dr. Frank Schaeffel Prof. Dr. Dr. Uwe IIg Prof. Dr. Siegfried Wahl Prof. Dr. Felix Wichmann 1 I hereby declare that I have produced the work entitled “Adaptations of the human eye to reduce the impact of chromatic aberration on vision”, submitted for the award of a doctorate, on my own (without external help), have used only the sources and aids indicated and have marked passages included from other works, whether verbatim or in content, as such. I swear upon oath that these statements are true and that I have not concealed anything. I am aware that making a false declaration under oath is punishable by a term of imprisonment of up to three years or by a fine. Tübingen, ......................................... ............................................................. Date Signature 2 To my parents 3 Contents 1. Acknowlegements ............................................................................................................... -

Stereoscopic Surface Interpolation from Illusory
STEREOSCOPIC SURFACE INTERPOLATION FROM ILLUSORY CONTOURS BRITTNEY HARTLE A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS GRADUATE PROGRAM IN PSYCHOLOGY YORK UNIVERSITY TORONTO, ONTARIO September 2016 © Brittney Hartle, 2016 Abstract Stereoscopic Kanizsa figures are an example of stereoscopic interpolation of an illusory surface. In such stimuli, luminance-defined disparity signals exist only along the edges of inducing elements, but observers reliably perceive a coherent surface that extends across the central region in depth. The aim of this series of experiments was to understand the nature of the disparity signal that underlies the perception of illusory stereoscopic surfaces. I systematically assessed the accuracy and precision of suprathreshold depth percepts using a collection of Kanizsa figures with a wide range of 2D and 3D properties. For comparison, I assessed similar perceptually equated figures with luminance-defined surfaces, with and without inducing elements. A cue combination analysis revealed that observers rely on ordinal depth cues in conjunction with stereopsis when making depth judgements. Thus, 2D properties (e.g. occlusion features and luminance relationships) contribute rich information about 3D surface structure by influencing perceived depth from binocular disparity. ii Table of Contents Abstract ........................................................................................................................................................ -

Visual Illusions
VISUAL ILLUSIONS: PERCEPTION OF LUMINANCE, COLOR, AND MOTION IN HUMANS Inaugural-Dissertation zur Erlangung des akademischen Grades Doctor rerum naturalium (Dr. rer. nat.) an der Justus-Liebig-Universität Giessen Fachbereich 06: Psychologie und Sportwissenschaften Otto-Behaghel-Strasse 10F 35394 Giessen vorgelegt am 21. Dezember 2006 von Dipl. Psych. Kai Hamburger geboren am 5. Juni 1977 in Gedern 1. Berichterstatter und Betreuer Prof. Karl R. Gegenfurtner, Ph.D. (Psychologie, Giessen) 2. Berichterstatter Prof. Dr. Hans Irtel (Psychologie, Mannheim) To my grandfather Heinrich, my parents Elke and Rainer, my brother Sven, and to my fiancée Sandra. Acknowledgement First of all, I would like to express my gratitude to my godfather in the graduate program, Professor Karl R. Gegenfurtner (Giessen, Germany), and my two other supervisors, Professor Lothar Spillmann (Freiburg, Germany) and Professor Arthur G. Shapiro (Lewisburg, PA, U.S.A.). Karl, on very short notice you gave me the opportunity to join the graduate program ‘Neural Representation and Action Control – Neuroact’ and by doing so one of the best departments in the field of Vision Sciences. I became a member of an extraordinary lab, which still excites me. You allowed me to finish projects which were already in progress when I started in Giessen and you gave me plenty of rope to pursue my own interests. Thus, I was able to publish efficiently and furthermore gained deep insights into the field of Vision Sciences and even beyond. This was the best mentoring a natural scientist could think of. Thank you. Professor Spillmann, you paved my way into the Vision Sciences. At the beginning of my scientific career you gave me the opportunity to join your famous ‘Freiburg Psychophysics Laboratory’. -

Anomalous Induction of Brightness and Surface Qualities: a New Illusion Due to Radial Lines and Chromatic Rings
Perception, 2003, volume 32, pages 1289 ^ 1305 DOI:10.1068/p3475 Anomalous induction of brightness and surface qualities: A new illusion due to radial lines and chromatic rings Baingio Pinnaô ½, Lothar Spillmannô, John S Werner# ô A G Hirnforschung, Universita« t Freiburg, Hansastrasse 9, D 79104 Freiburg, Germany; # Department of Ophthalmology and Section of Neurobiology, Physiology & Behavior, University of California, Davis, 4860 Y Street, Suite 2400 Sacramento, CA 95817, USA; e-mail: [email protected] Received 16 October 2002, in revised form 5 June 2003; published online 10 December 2003 Abstract. When a chromatic (eg light-blue) annulus surrounds the central gap of an Ehrenstein figure so as to connect the inner ends of the radial lines, a striking new lightness effect emerges: the central white disk has both a self-luminous quality (brighter than in the regular Ehrenstein figure) and a surface quality (dense, paste-like). Self-luminous and surface qualities do not ordinarily appear co-extensively: hence, the brightness induction is called anomalous. In experi- ment 1, subjects separately scaled self-luminous and surface properties, and in experiment 2, brightness was nulled by physically darkening the central gap. Experiments 3 and 4 were designed to evaluate the importance of chromatic versus achromatic properties of the annulus; other aspects of the annulus (width or the inclusion of a thin black ring inside or outside the chromatic annulus) were tested in experiments 5 ^ 7. In experiments 8 ^ 12, subjects rated the brightness of modified Ehrenstein figures varying the radial lines (number, length, width, contrast, arrangement). Variation of these parameters generally affected brightness enhancement in the Ehrenstein figure and anomalous brightness induction in a similar manner, but was stronger for the latter effect.