Doxycycline Hyclate) Capsule, Delayed Release Pellets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of Nanobiosensors in Therapeutic Drug Monitoring
Journal of Personalized Medicine Review Personalized Medicine for Antibiotics: The Role of Nanobiosensors in Therapeutic Drug Monitoring Vivian Garzón 1, Rosa-Helena Bustos 2 and Daniel G. Pinacho 2,* 1 PhD Biosciences Program, Universidad de La Sabana, Chía 140013, Colombia; [email protected] 2 Therapeutical Evidence Group, Clinical Pharmacology, Universidad de La Sabana, Chía 140013, Colombia; [email protected] * Correspondence: [email protected]; Tel.: +57-1-8615555 (ext. 23309) Received: 21 August 2020; Accepted: 7 September 2020; Published: 25 September 2020 Abstract: Due to the high bacterial resistance to antibiotics (AB), it has become necessary to adjust the dose aimed at personalized medicine by means of therapeutic drug monitoring (TDM). TDM is a fundamental tool for measuring the concentration of drugs that have a limited or highly toxic dose in different body fluids, such as blood, plasma, serum, and urine, among others. Using different techniques that allow for the pharmacokinetic (PK) and pharmacodynamic (PD) analysis of the drug, TDM can reduce the risks inherent in treatment. Among these techniques, nanotechnology focused on biosensors, which are relevant due to their versatility, sensitivity, specificity, and low cost. They provide results in real time, using an element for biological recognition coupled to a signal transducer. This review describes recent advances in the quantification of AB using biosensors with a focus on TDM as a fundamental aspect of personalized medicine. Keywords: biosensors; therapeutic drug monitoring (TDM), antibiotic; personalized medicine 1. Introduction The discovery of antibiotics (AB) ushered in a new era of progress in controlling bacterial infections in human health, agriculture, and livestock [1] However, the use of AB has been challenged due to the appearance of multi-resistant bacteria (MDR), which have increased significantly in recent years due to AB mismanagement and have become a global public health problem [2]. -

National Antibiotic Consumption for Human Use in Sierra Leone (2017–2019): a Cross-Sectional Study
Tropical Medicine and Infectious Disease Article National Antibiotic Consumption for Human Use in Sierra Leone (2017–2019): A Cross-Sectional Study Joseph Sam Kanu 1,2,* , Mohammed Khogali 3, Katrina Hann 4 , Wenjing Tao 5, Shuwary Barlatt 6,7, James Komeh 6, Joy Johnson 6, Mohamed Sesay 6, Mohamed Alex Vandi 8, Hannock Tweya 9, Collins Timire 10, Onome Thomas Abiri 6,11 , Fawzi Thomas 6, Ahmed Sankoh-Hughes 12, Bailah Molleh 4, Anna Maruta 13 and Anthony D. Harries 10,14 1 National Disease Surveillance Programme, Sierra Leone National Public Health Emergency Operations Centre, Ministry of Health and Sanitation, Cockerill, Wilkinson Road, Freetown, Sierra Leone 2 Department of Community Health, Faculty of Clinical Sciences, College of Medicine and Allied Health Sciences, University of Sierra Leone, Freetown, Sierra Leone 3 Special Programme for Research and Training in Tropical Diseases (TDR), World Health Organization, 1211 Geneva, Switzerland; [email protected] 4 Sustainable Health Systems, Freetown, Sierra Leone; [email protected] (K.H.); [email protected] (B.M.) 5 Unit for Antibiotics and Infection Control, Public Health Agency of Sweden, Folkhalsomyndigheten, SE-171 82 Stockholm, Sweden; [email protected] 6 Pharmacy Board of Sierra Leone, Central Medical Stores, New England Ville, Freetown, Sierra Leone; [email protected] (S.B.); [email protected] (J.K.); [email protected] (J.J.); [email protected] (M.S.); [email protected] (O.T.A.); [email protected] (F.T.) Citation: Kanu, J.S.; Khogali, M.; 7 Department of Pharmaceutics and Clinical Pharmacy & Therapeutics, Faculty of Pharmaceutical Sciences, Hann, K.; Tao, W.; Barlatt, S.; Komeh, College of Medicine and Allied Health Sciences, University of Sierra Leone, Freetown 0000, Sierra Leone 8 J.; Johnson, J.; Sesay, M.; Vandi, M.A.; Directorate of Health Security & Emergencies, Ministry of Health and Sanitation, Sierra Leone National Tweya, H.; et al. -

Sexually Transmitted Diseases Treatment Options
Sexually transmitted disease (STD) treatment options PREFERRED & ALTERNATIVE OPTIONS Many clinical partners are operating in a limited capacity during the COVID-19 pandemic. Below are preferred (in clinic or other location where injections can be given) and alternative (when only oral medicines are available 1) treatments for STDs. Syndrome Preferred Treatments Alternative Treatments Follow-up Male urethritis syndrome Ceftriaxone 250mg intramuscular (IM) x 1 PLUS Men who have sex with men (MSM) and transgender women2: Patients should be counseled to azithromycin 1g PO x 1 Cefixime 800 mg PO x 1 PLUS doxycycline 100 mg PO BID x 7 days be tested for STDs once clinical Presumptively treating: care is resumed in the local If azithromycin is not available: doxycycline 100 Men who have sex with women only: gonorrhea clinics. Clients who have been mg PO BID for 7 days (except in pregnancy3) Cefixime 800mg PO x 1 PLUS azithromycin 1g PO x 1 referred for oral treatment If cephalosporin allergy5 is reported, gentamicin If cefixime is unavailable, substitute cefpodoxime 400mg PO q12h should return for 240mg IM x 1 PLUS azithromycin 2g PO x 1 x 2 for cefixime in above regimens4 comprehensive testing and screening and linked to services If oral cephalosporin not available or history of cephalosporin at that time. allergy5: azithromycin 2g PO x 1 If azithromycin is not available: doxycycline 100 mg PO BID for 7 days (except in pregnancy3) Patients should be advised to abstain from sex for 7 days Treatment typically guided by examination and For presumptive therapy when examination and laboratory following completion of Vaginal discharge syndrome treatment. -

Doxycycline/Minocycline Step Therapy Criteria Program Summary
Doxycycline/Minocycline Step Therapy Criteria Program Summary This criteria applies to Commercial, NetResults F series and Health Insurance Marketplace formularies. OBJECTIVE The intent of the Doxycycline/Minocycline Step Therapy (ST) program is to encourage the use of both cost-effective preferred doxycycline and minocycline products prior to the use of nonpreferred brand or generic doxycycline or minocycline products and to accommodate for use of brand nonpreferred doxycycline or minocycline products when the generic products cannot be used due to previous trial, documented intolerance, FDA labeled contraindication, or hypersensitivity. Requests for nonpreferred doxycycline and minocycline products will be reviewed when patient-specific documentation has been provided. TARGET DRUGS Doxycycline Products: Acticlate™ (doxycycline hyclate tablet) Adoxa (doxycycline monohydrate tablet, capsule) Doryx, Doryx MPC® (doxycycline hyclate delayed-release tablet)a Doxycycline (doxycycline hyclate delayed-release capsulea, doxycycline hyclate tablet, doxycycline monohydrate delayed release capsule) Monodox (doxycycline monohydrate capsule) Oracea (doxycycline monohydrate delayed-release capsule) Vibramycin (doxycycline hyclate capsule, doxycycline monohydrate suspension, doxycycline calcium syrup Minocycline Products: Minocin (minocycline capsule) minocycline tablet Solodyn (minocycline extended-release tablet)a a - available as a generic; designated target PRIOR AUTHORIZATION CRITERIA FOR APPROVAL Brand and Nonpreferred generic doxycycline and minocycline products will be approved when BOTH of the following are met: 1. ONE of the following: a. The patient’s medication history includes use of a preferred generic doxycycline product OR b. The patient has a documented intolerance, FDA labeled contraindication, or hypersensitivity to a preferred generic doxycycline product AND 2. ONE of the following is met: a. The patient’s medication history includes use of a preferred generic minocycline product OR b. -

Third ESVAC Report
Sales of veterinary antimicrobial agents in 25 EU/EEA countries in 2011 Third ESVAC report An agency of the European Union The mission of the European Medicines Agency is to foster scientific excellence in the evaluation and supervision of medicines, for the benefit of public and animal health. Legal role Guiding principles The European Medicines Agency is the European Union • We are strongly committed to public and animal (EU) body responsible for coordinating the existing health. scientific resources put at its disposal by Member States • We make independent recommendations based on for the evaluation, supervision and pharmacovigilance scientific evidence, using state-of-the-art knowledge of medicinal products. and expertise in our field. • We support research and innovation to stimulate the The Agency provides the Member States and the development of better medicines. institutions of the EU the best-possible scientific advice on any question relating to the evaluation of the quality, • We value the contribution of our partners and stake- safety and efficacy of medicinal products for human or holders to our work. veterinary use referred to it in accordance with the • We assure continual improvement of our processes provisions of EU legislation relating to medicinal prod- and procedures, in accordance with recognised quality ucts. standards. • We adhere to high standards of professional and Principal activities personal integrity. Working with the Member States and the European • We communicate in an open, transparent manner Commission as partners in a European medicines with all of our partners, stakeholders and colleagues. network, the European Medicines Agency: • We promote the well-being, motivation and ongoing professional development of every member of the • provides independent, science-based recommenda- Agency. -

Trend Analysis of Antibiotics Consumption Using WHO Aware Classification in Tertiary Care Hospital
International Journal of Basic & Clinical Pharmacology Bhardwaj A et al. Int J Basic Clin Pharmacol. 2020 Nov;9(11):1675-1680 http:// www.ijbcp.com pISSN 2319-2003 | eISSN 2279-0780 DOI: https://dx.doi.org/10.18203/2319-2003.ijbcp20204493 Original Research Article Trend analysis of antibiotics consumption using WHO AWaRe classification in tertiary care hospital Ankit Bhardwaj*, Kaveri Kapoor, Vivek Singh Saraswathi institute of Medical Sciences, Pilakhuwa, Hapur, Uttar Pradesh, India Received: 21 August 2020 Accepted: 21 September 2020 *Correspondence: Dr. Ankit Bhardwaj, Email: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Aim of the study was to assess trend in antibiotics consumption pattern from 2016 to 2019 using AWaRe classification, ATC and Defined daily dose methodology (DDD) in a tertiary care hospital. Antibiotics are crucial for treating infectious diseases and have significantly improved the prognosis of patients with infectious diseases, reducing morbidity and mortality. The aim of the study is to classify the antibiotic based on WHO AWaRe classification and compare their four-year consumption trends. The study was conducted at a tertiary care center, Pilakhuwa, Hapur. Antibiotic procurement data for a period of 4 years (2016-2019) was collected from the Central medical store. Methods: This is a retrospective time series analysis of systemic antibiotics with no intervention at patient level. Antibiotic procurement was taken as proxy for consumption assuming that same has been used. -

Estonian Statistics on Medicines 2013 1/44
Estonian Statistics on Medicines 2013 DDD/1000/ ATC code ATC group / INN (rout of admin.) Quantity sold Unit DDD Unit day A ALIMENTARY TRACT AND METABOLISM 146,8152 A01 STOMATOLOGICAL PREPARATIONS 0,0760 A01A STOMATOLOGICAL PREPARATIONS 0,0760 A01AB Antiinfectives and antiseptics for local oral treatment 0,0760 A01AB09 Miconazole(O) 7139,2 g 0,2 g 0,0760 A01AB12 Hexetidine(O) 1541120 ml A01AB81 Neomycin+Benzocaine(C) 23900 pieces A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+Thymol(dental) 2639 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+Cetylpyridinium chloride(gingival) 179340 g A01AD81 Lidocaine+Cetrimide(O) 23565 g A01AD82 Choline salicylate(O) 824240 pieces A01AD83 Lidocaine+Chamomille extract(O) 317140 g A01AD86 Lidocaine+Eugenol(gingival) 1128 g A02 DRUGS FOR ACID RELATED DISORDERS 35,6598 A02A ANTACIDS 0,9596 Combinations and complexes of aluminium, calcium and A02AD 0,9596 magnesium compounds A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 591680 pieces 10 pieces 0,1261 A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 1998558 ml 50 ml 0,0852 A02AD82 Aluminium aminoacetate+Magnesium oxide(O) 463540 pieces 10 pieces 0,0988 A02AD83 Calcium carbonate+Magnesium carbonate(O) 3049560 pieces 10 pieces 0,6497 A02AF Antacids with antiflatulents Aluminium hydroxide+Magnesium A02AF80 1000790 ml hydroxide+Simeticone(O) DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 34,7001 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 3,5364 A02BA02 Ranitidine(O) 494352,3 g 0,3 g 3,5106 A02BA02 Ranitidine(P) -

WHO Report on Surveillance of Antibiotic Consumption: 2016-2018 Early Implementation ISBN 978-92-4-151488-0 © World Health Organization 2018 Some Rights Reserved
WHO Report on Surveillance of Antibiotic Consumption 2016-2018 Early implementation WHO Report on Surveillance of Antibiotic Consumption 2016 - 2018 Early implementation WHO report on surveillance of antibiotic consumption: 2016-2018 early implementation ISBN 978-92-4-151488-0 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution- NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons. org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non- commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. WHO report on surveillance of antibiotic consumption: 2016-2018 early implementation. Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) Sales Data and Animal Population Data Reporting Protocol (Version 4)
9 March 2021 EMA/210691/2015 Rev. 4 Veterinary Medicines Division European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) Sales Data and Animal Population Data Reporting Protocol (version 4) Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us An agency of the European Union Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 © European Medicines Agency, 2021. Reproduction is authorised provided the source is acknowledged. Table of content 1. Introduction ............................................................................................ 3 1.1. Terms of reference ............................................................................................. 3 1.2. Approach .......................................................................................................... 4 1.3. Organization of the project .................................................................................. 4 1.4. ESVAC web-based application .............................................................................. 5 2. ESVAC sales data ..................................................................................... 5 2.1. Selection of data source ...................................................................................... 5 2.2. Antimicrobial substances used in veterinary medicine to be included in sales dataset ... 6 2.3. Variables to be collected for each VMP presentation ............................................... -

Common Study Protocol for Observational Database Studies WP5 – Analytic Database Studies
Arrhythmogenic potential of drugs FP7-HEALTH-241679 http://www.aritmo-project.org/ Common Study Protocol for Observational Database Studies WP5 – Analytic Database Studies V 1.3 Draft Lead beneficiary: EMC Date: 03/01/2010 Nature: Report Dissemination level: D5.2 Report on Common Study Protocol for Observational Database Studies WP5: Conduct of Additional Observational Security: Studies. Author(s): Gianluca Trifiro’ (EMC), Giampiero Version: v1.1– 2/85 Mazzaglia (F-SIMG) Draft TABLE OF CONTENTS DOCUMENT INFOOMATION AND HISTORY ...........................................................................4 DEFINITIONS .................................................... ERRORE. IL SEGNALIBRO NON È DEFINITO. ABBREVIATIONS ......................................................................................................................6 1. BACKGROUND .................................................................................................................7 2. STUDY OBJECTIVES................................ ERRORE. IL SEGNALIBRO NON È DEFINITO. 3. METHODS ..........................................................................................................................8 3.1.STUDY DESIGN ....................................................................................................................8 3.2.DATA SOURCES ..................................................................................................................9 3.2.1. IPCI Database .....................................................................................................9 -

Review of Existing Classification Efforts
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.1.1 Review of existing classification efforts Due date of deliverable: (15.01.2008) Actual submission date: (07.02.2008) Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UGent Revision 1.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public X PP Restricted to other programme participants (including the Commission Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) Task 4.1 : Review of existing classification efforts Authors: Kristof Pil, Elke Raes, Thomas Van den Neste, An-Sofie Goessaert, Jolien Veramme, Alain Verstraete (Ghent University, Belgium) Partners: - F. Javier Alvarez (work package leader), M. Trinidad Gómez-Talegón, Inmaculada Fierro (University of Valladolid, Spain) - Monica Colas, Juan Carlos Gonzalez-Luque (DGT, Spain) - Han de Gier, Sylvia Hummel, Sholeh Mobaser (University of Groningen, the Netherlands) - Martina Albrecht, Michael Heiβing (Bundesanstalt für Straßenwesen, Germany) - Michel Mallaret, Charles Mercier-Guyon (University of Grenoble, Centre Regional de Pharmacovigilance, France) - Vassilis Papakostopoulos, Villy Portouli, Andriani Mousadakou (Centre for Research and Technology Hellas, Greece) DRUID 6th Framework Programme Deliverable D.4.1.1. Revision 1.0 Review of Existing Classification Efforts Page 2 of 127 Introduction DRUID work package 4 focusses on the classification and labeling of medicinal drugs according to their influence on driving performance. -
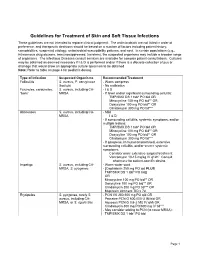
Guidelines for Treatment of Skin and Soft Tissue Infections These Guidelines Are Not Intended to Replace Clinical Judgment
Guidelines for Treatment of Skin and Soft Tissue Infections These guidelines are not intended to replace clinical judgment. The antimicrobials are not listed in order of preference, and therapeutic decisions should be based on a number of factors including patient history, comorbidities, suspected etiology, antimicrobial susceptibility patterns, and cost. In certain populations (e.g., intravenous drug abusers, immunosuppressed, travelers), the suspected organisms may include a broader range of organisms. The Infectious Diseases consult services are available for complex patient consultations. Cultures may be obtained as deemed necessary if I & D is performed and/or if there is a discrete collection of pus or drainage that would allow an appropriate culture specimen to be obtained. Note: Refer to table on page 4 for pediatric dosing. Type of Infection Suspected Organisms Recommended Treatment Folliculitis S. aureus, P. aeruginosa - Warm compress (hot tub) - No antibiotics Furuncles, carbuncles, S. aureus, including CA- - I & D “boils” MRSA - If fever and/or significant surrounding cellulitis: TMP/SMX DS 1 tab* PO bid OR Minocycline 100 mg PO bid** OR Doxycyline 100 mg PO bid** OR Clindamycin 300 mg PO tid*** Abscesses S. aureus, including CA- - Mild MRSA I & D - If surrounding cellulitis, systemic symptoms, and/or multiple lesions: TMP/SMX DS 1 tab* PO bid OR Minocycline 100 mg PO bid** OR Doxycyline 100 mg PO bid** OR Clindamycin 300 mg PO tid*** - If gangrene, immunocompromised, extensive surrounding cellulitis, and/or severe systemic symptoms: Consider more extensive surgical treatment Vancomycin 10-15 mg/kg IV q12h§ Consult pharmacy for patient-specific dosing. Impetigo S. aureus, including CA- - Warm water soak MRSA, S.