Dyspnoea in Advanced Disease Oxford University Press Makes No Representation, Express Or Implied, That the Drug Dosages in This Book Are Correct
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CARDINAL SYMPTOMS of HEART DISEASE Exam 1 | Dr. Donato Marañon | September 24, 2012
OS 213: Cardiovascular System LEC 02: CARDINAL SYMPTOMS OF HEART DISEASE Exam 1 | Dr. Donato Marañon | September 24, 2012 OUTLINE Common Cardiac Symptomatology B. Dyspnea A. Chest Pain C. Palpitations 1. Attributes of Pain D. Edema 2. Definitions E. Cyanosis 3. Chronic Recurrent Chest Pain Syndrome F. Syncope 4. Acute Chest Pain Syndrome II. Importance of History and PE 5. Case Discussion The lecture is similar from block B’s Lecture but we changed the formatting though kasing ang gulo nung topic. So if mas naguluhan kayo sorry. Some changes: 1. All the symptomatology are now under common cardiac symptomatology. 2. Differentials for chest pain have been divided into chronic and acute. I. COMMON CARDIAC SYMPTOMATOLOGY Symptoms: complaints of the patient (most common complaint: pain); Includes chest pain, dyspnea, palpitations, edema, cyanosis, syncope Signs: doctors’ objective findings and observations A. CHEST PAIN Chest pain: most common but not exhaustive - can be caused by other factors such as hypertension ATTRIBUTES OF PAIN (PPQRSTO) Provocative – what provokes/triggers the pain o Is it precipitated by effort (exertional)? o At what time does it appear? When you are trying to get up, moving the body, etc… Palliative – what relieves/palliates the pain o Medications, therapy, etc… Quality – the nature of the pain o Sharp, burning, pricking, stabbing, strangulating, oppressing, ache similar to muscle ache, etc. Region/Radiation – location (primary region where the pain is felt), central region, how wide the coverage is and where the pain radiates or is worst o Central precordial pain where does it radiate? Back? Leg? o Hard to interview Filipinos – “doon, diyan” – vague descriptions of location Severity – intensity: mild, moderate, severe o May use a scale from 0 to 10 (worst) o Give open-ended questions Timing o Onset – abrupt, worse at start, insidious, builds up/gradual Ian, Aca, Hannah UPCM 2016A: XVI, Walang 1 of Kapantay! 14 OS 213: Cardiovascular System LEC 02: CARDINAL SYMPTOMS OF HEART DISEASE Exam 1 | Dr. -

Orthopnea : Dyspnoea on Supine Position
PRESENTED BY Dr . G . Subrahmanyam M.D., D.M., Professor of Cardiology Director of Narayana Medical Institutions Ex-Professor of Cardiology SVMC &SVRRH Ex Asst. Professor of Medicine, SVMC & SVRRH S CSSCLASS RANKS BAGGED . DURING 2010 1 FIRST M.B.B.S 1ST, 2ND, 3RD &5TH 2 SECOND M. B. B. S 4TH, 5TH, 6TH &10& 10TH 3 THIRD PART-I 1ST & 9TH 4 THRID PART-II 5th, 8th, 10TH NARAYANA NURSING INSTITUTION S.NO RANKS OBTAINED IN M.Sc(Nursing) during 2010 11st,2nd,3rd, 4th,5th, 6th,7th, 8th NARAYANA PHARMACY COLLEGE NARAYANA DENTAL COLLEGE • In MDS results, 27 out of 28 students passed successfully. • MDS(Prostodontics) is the topper in the University wide during 2010. • The only centre in AP with all the Superspecialities like M. CH( Surgical gastro enterology). • History will give you likely diagnosis over 75% of the time • DO NOT SKIP IT in favour of tests • History will help you immediately – Test s will ta ke time to come bac k an d may resu lt in more questions than answers. Best in the physician’s Quer History is the richest source of information Patient spouse gives good information (Chyne stokes respiration) His tory : 1G1. Genera lMdilHitl Medical History 2. Personal and past history 3. Occupational history 4. Nutritional history DYSPNOEA • Dyspnoea on Exercise • Dyspnoea on deconditioning Normal person. But moderate exercise unaccumastomed causes Dyspnoea • Interstial and alveolar oedema stretches ‘J’ receptors in the lung (CCF) ↓cardiac output (TOF) without lung congestion. Inspiratory Dyspnoea : Obstructive airway disease. Expiratory Dyspnoea: Obstruction to lower airways Exertional Dyspnoea : COPD, Cardiac failure Dyspnoea developing at rest Pheumothorax PlPul.em blibolism Dyspnoea occuring at rest and absent on exertion : Functional DYSPNOEA Cardiac, Renal, Ovarian, Bronchial, Psychogenic Postural – Myxoma Squatting ↓ Tof Trepopnea : Lateral position occurs eg. -

Redalyc.POSTERS EXPOSTOS
Revista Portuguesa de Pneumología ISSN: 0873-2159 [email protected] Sociedade Portuguesa de Pneumologia Portugal POSTERS EXPOSTOS Revista Portuguesa de Pneumología, vol. 23, núm. 3, noviembre, 2017 Sociedade Portuguesa de Pneumologia Lisboa, Portugal Disponível em: http://www.redalyc.org/articulo.oa?id=169753668003 Como citar este artigo Número completo Sistema de Informação Científica Mais artigos Rede de Revistas Científicas da América Latina, Caribe , Espanha e Portugal Home da revista no Redalyc Projeto acadêmico sem fins lucrativos desenvolvido no âmbito da iniciativa Acesso Aberto Document downloaded from http://www.elsevier.es, day 06/12/2017. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. POSTERS EXPOSTOS PE 001 PE 002 A PLEASANT FINDING MALIGNANT CHEST PAIN A Pais, AI Coutinho, M Cardoso, A Pignatelli, C Bárbara A Pais, C Pereira, C Antunes, V Pereira, AI Coutinho, A Feliciano, Centro Hospitalar de Lisboa Norte C Quadros, A Ribeiro, L Carvalho, C Bárbara Centro Hospitalar de Lisboa Norte Key-words: mass, debridement, hamartoma Key-words: pain, S100, sarcoma 37-year-old male patient, salesman. Sporadic smoker. With a past history of allergic rhinitis and chronic gastritis. With no usual 26-year-old male patient, supermarket employee. Smoker of 10 medication. In January 2017, he was diagnosed with a respiratory pack-year. Past history of bronchial asthma in childhood. No rel - infection, having completed ten days of empirical antibiotic ther - evant family history. Without usual ambulatory medication. With apy with amoxicillin / clavulanic acid, with clinical improvement. a history of dry cough and chest pain in the posterior region of In May 2017, he underwent thoracic xray, which revealed homoge - the left hemithorax, for about two years, having had at that time, neous opacity of triangular morphology in the middle lobe of the chest X-ray without pathological findings, and the clinical picture right lung. -

Chapter 11 Dyspnea, Orthopnea, and Paroxysmal Nocturnal Dyspnea
2/12/2015 Dyspnea, Orthopnea, and Paroxysmal Nocturnal Dyspnea Clinical Methods NCBI Bookshelf NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 11 Dyspnea, Orthopnea, and Paroxysmal Nocturnal Dyspnea Vaskar Mukerji. Definition Dyspnea refers to the sensation of difficult or uncomfortable breathing. It is a subjective experience perceived and reported by an affected patient. Dyspnea on exertion (DOE) may occur normally, but is considered indicative of disease when it occurs at a level of activity that is usually well tolerated. Dyspnea should be differentiated from tachypnea, hyperventilation, and hyperpnea, which refer to respiratory variations regardless of the patients" subjective sensations. Tachypnea is an increase in the respiratory rate above normal; hyperventilation is increased minute ventilation relative to metabolic need, and hyperpnea is a disproportionate rise in minute ventilation relative to an increase in metabolic level. These conditions may not always be associated with dyspnea. Orthopnea is the sensation of breathlessness in the recumbent position, relieved by sitting or standing. Paroxysmal nocturnal dyspnea (PND) is a sensation of shortness of breath that awakens the patient, often after 1 or 2 hours of sleep, and is usually relieved in the upright position. Two uncommon types of breathlessness are trepopnea and platypnea. Trepopnea is dyspnea that occurs in one lateral decubitus position as opposed to the other. Platypnea refers to breathlessness that occurs in the upright position and is relieved with recumbency. Technique A patient with dyspnea may say: "I feel short of breath," "I"m having difficulty breathing," "I can"t catch my breath," "I feel like I"m suffocating." Because it is a subjective phenomenon, the perception of dyspnea and its interpretation vary from patient to patient. -

Subtle Radiographic Presentation of a Pleural Effusion Secondary to A
ISSN 0008-3194 (p)/ISSN 1715-6181 (e)/2014/273–279/$2.00/©JCCA 2014 Subtle radiographic presentation of a pleural effusion secondary to a cancer of unknown primary: a case study Marc-André Blanchette, DC, MSc1 Julie-Marthe Grenier, DC, DACBR/FCCR(C)2 Carcinoma of unknown primary sites is a clinical Les cancers de sites primaires inconnus représentent syndrome that represents many types of cancer. The un syndrome clinique englobant de nombreux types mortality rate associate to this type of cancer is de néoplasie. Le taux de mortalité associé à ce type elevated and a rapid medical referral is required for de cancer est élevé et une consultation médicale patients presenting this condition. Pleural effusion may rapide est nécessaire chez les patients présentant cette be the only visible sign. We report a case of pleural affection. Un épanchement pleural peut être le seul effusion secondary to a cancer of unknown primary signe radiographique visible. Nous rapportons un site in a 60-year-old man that sought chiropractic cas d’épanchement pleural secondaire à un cancer care for radiating low back pain. The radiographic de sites primaires inconnus chez un homme de 60 ans studies revealed a pleural effusion as one of the only qui consultait en chiropratique pour une lombalgie significant finding. This article will address the clinical irradiante. Les études radiographiques ont révélé un presentation, radiographic studies and a discussion on épanchement pleural comme une trouvaille fortuite. the radiographic detection of pleural effusion. Nous avons inclus la présentation clinique, les examens radiographiques et une discussion sur la détection d’un épanchement pleural. -

Physical Diagnosis the Pulmonary Exam What Should We Know About the Examination of the Chest?
PHYSICAL DIAGNOSIS THE PULMONARY EXAM WHAT SHOULD WE KNOW ABOUT THE EXAMINATION OF THE CHEST? • LANDMARKS • PERTINENT VOCABULARY • SYMPTOMS • SIGNS • HOW TO PERFORM AN EXAM • HOW TO PRESENT THE INFORMATION • HOW TO FORMULATE A DIFFERENTIAL DIAGNOSIS IMPORTANT TOPOGRAPHY OF THE CHEST TOPOGRAPHY OF THE BACK LOOK AT THE PATIENT • RESPIRATORY DISTRESS • ANXIOUS • CLUTCHING • ACCESSORY MUSCLES •CYANOSIS • GASPING • STRIDOR • CLUBBING TYPES OF BODY HABITUS WHAT IS A BARRELL CHEST? • THORACIC INDEX – RATIO OF THE ANTERIORPOSTERIOR TO LATERAL DIAMETER NORMAL 0.70 – 0.75 IN ADULTS - >0.9 IS CONSIDERED ABNORMAL • NORMALS - ILLUSION •COPD AM J MED 25:13-22,1958 PURSED – LIPS BREATHING • COPD – DECREASES DYSPNEA • DECREASES RR • INCREASES TIDAL VOLUME • DECREASES WORK OF BREATHING CHEST 101:75-78, 1992 WHITE NOISE (NOISY BREATHING) • THIS NOISE CAN BE HEARD AT THE BEDSIDE WITHOUT THE STETHOSCOPE • LACKS A MUSICAL PITCH • AIR TURBULENCE CAUSED BY NARROWED AIRWAYS • CHRONIC BRONCHITIS CHEST 73:399-412, 1978 RESPIRATORY ALTERNANS • NORMALLY BOTH CHEST AND ABDOMEN RISE DURING INSPIRATION • PARADOXICAL RESPIRATION IMPLIES THAT DURING INSPIRATION THE CHEST RISES AND THE ABDOMEN COLLAPSES • IMPENDING MUSCLE FATIGUE DO NOT FORGET THE TRACHEA • TRACHEAL DEVIATION • AUSCULTATE - STRIDOR • TRACHEAL TUG (OLIVERS SIGN) – DOWNWARD DISPLACEMENT OF THE CRICOID CARTILAGE WITH VENTRICULAR CONTRACTION – OBSERVED IN PATIENTS WITH AN AORTIC ARCH ANEURYSM • TRACHEAL TUG (CAMPBELL’S SIGN) – DOWNWARD DISPACEMENT OF THE THYROID CARTILAGE DURING INSPIRATION – SEEN IN PATIENTS -

Surgical Ventricular Remodeling in Ischemic Heart Failure: the Impact of Optimal Volume Reduction on Long-Term Outcome
UNIVERSITY OF GENOA School of Medical and Pharmaceutical Sciences Master’s degree course in Medicine and Surgery DEGREE THESIS SURGICAL VENTRICULAR REMODELING IN ISCHEMIC HEART FAILURE: THE IMPACT OF OPTIMAL VOLUME REDUCTION ON LONG-TERM OUTCOME SUPERVISOR CANDIDATE Francesco Santini, M.D. Francesca Zanin CO-SUPERVISORS Antonio Salsano, M.D. Serenella Castelvecchio, M.D. Lorenzo Menicanti, M.D. Academic Year 2019-2020 Ai miei genitori, i miei punti cardinali 2 INDEX 1. Introduction ..................................................................................................... 6 2. Heart Failure .................................................................................................. 10 2.1. Definition and classification ................................................................................ 10 2.2. Epidemiology and Impact on the population ..................................................... 15 2.3. Etiology .................................................................................................................. 17 2.3.1. Ischemic etiology .................................................................................................. 19 2.4. Pathophysiology ................................................................................................... 22 2.4.1. Left Ventricular Remodeling ................................................................................. 25 2.5. Diagnosis .............................................................................................................. -

数字 Accessory Bronchus 副気管支 Accessory Fissure 副葉間裂
数字 accentuation 亢進 accessory 副の 数字 accessory bronchus 副気管支 accessory fissure 副葉間裂 10-year survival 10年生存 accessory lobe 副肺葉 18F-fluorodeoxy glucose (FDG) 18F-フルオロデオキシグルコース accessory lung 副肺 2,3-diphosphoglycerate (2,3-DPG) 2,3ジフォスフォグリセレート accessory nasal sinus 副鼻腔 201TI (thallium-201) タリウム accessory trachea 副気管 5-fluorouracil(FU) 5-フルオロウラシル acclimation 順化 5-HT3 receptor antagonist 5-HT3レセプター拮抗薬 acclimation 馴化 5-hydroxytryptamine 5-ヒドロオキシトリプタミン acclimatization 気候順応 5-year survival 5年生存 acclimatization 順化 99mTc-macroaggregated albumin (99mTc-MAA) 99mTc標識大 acclimatization 馴化 凝集アルブミン accommodation 順応 accommodation 調節 accommodation to high altitude 高所順(適)応 A ACE polymorphism ACE遺伝子多型 acetone body アセトン体 abdomen 腹部 acetonuria アセトン尿[症] abdominal 腹部[側]の acetylcholine(ACh) アセチルコリン abdominal breathing 腹式呼吸 acetylcholine receptor (AchR, AChR) アセチルコリン受容体(レセプ abdominal cavity 腹腔 ター) abdominal pressure 腹腔内圧 acetylcholinesterase (AchE, AChE) アセチルコリンエステラーゼ abdominal respiration 腹式呼吸 achalasia アカラシア abdominal wall reflex 腹壁反射 achalasia 弛緩不能症 abduction 外転 achalasia [噴門]無弛緩[症] aberrant 走性 achromatocyte (achromocyte) 無血色素[赤]血球 aberrant 迷入性 achromatocyte (achromocyte) 無へモグロビン[赤]血球 aberrant artery 迷入動脈 acid 酸 aberration 迷入 acid 酸性 ablation 剥離 acid base equilibrium 酸塩基平衡 abnormal breath sound(s) 異常呼吸音 acid fast 抗酸性の abortive 早産の acid fast bacillus 抗酸菌 abortive 頓挫性(型) acid-base 酸―塩基 abortive 不全型 acid-base balance 酸塩基平衡 abortive pneumonia 頓挫[性]肺炎 acid-base disturbance 酸塩基平衡異常 abrasion 剥離 acid-base equilibrium 酸塩基平衡 abscess 膿瘍 acid-base regulation 酸塩基調節 absolute -

Cardiac + Pulmonary Non Cardiac Non Pulmonary
DYSPNEA INTRODUCTION WHAT IS DYSPNOEA? Dyspnea derives from Greek for “hard breathing”. It is often also described as “shortness of breath”. This is a subjective sensation of breathing, from mild discomfort to feelings of suffocation. It is a sign of a variety of disorders and is primarily an indication of inadequate ventilation or of insufficient amounts of oxygen in the circulating blood. INTRODUCTION WHAT IS DYSPNOEA? Dyspnea derives from Greek for “hard breathing”. It is often also described as “shortness of breath”. This is a subjective sensation of breathing, from mild discomfort to feelings of suffocation. It is a sign of a variety of disorders and is primarily an indication of inadequate ventilation or of insufficient amounts of oxygen in the circulating blood. PATHOPHYSIOLOGY Dyspnea happens when a “mismatch” occurs between afferent and efferent signaling. As the brain receives afferent ventilation information, it is able to compare it to the current level of respiration by the efferent signals. If the level of respiration is inappropriate for the body’s status and need, then dyspnea might occur PATHOPHYSIOLOGY RECEPTORS AND SIGNALS The pathway that leads to dyspnea via specific acid-sensing ion channels, mechanoreceptors and lung receptors located in different zones of the respiratory apparatus. Chemoreceptors Muscle spindles in the chest wall In the carotid bodies and medulla Signals the stretch and tension of the supply information with regard to respiratory muscles the blood gas levels of O2, CO2 and H+ EFFERENT SIGNALS Juxtacapillary receptors Motor neuronal signals descending Sensitive to pulmonary interstitial to the respiratory muscles, the most oedema important being the diaphragm Stretch receptors Hering-breuer reflex PATHOPHYSIOLOGY PATHOPHYSIOLOGY PATHOPHYSIOLOGY Three main components contribute to dyspnea: afferent signals, efferent signals, and central information processing. -

Approach to the Respiratory Patient
APPROACH TO THE RESPIRATORY PATIENT Differential Diagnosis of Dyspnea Differential Diagnosis of Cough Differential Diagnosis of Hemoptysis dyspnea/SOB • orthopnea: SOB when recumbent in CHF, asthma, COPD, or GERD • trepopnea: SOB when right or LLD position in CHF, cardiac mass • platypnea: SOB when upright in post-pneumonectomy, neurologic disease, hepatopulmonary syndrome (ie. liver failure), hypovolemia • episodic: in bronchospasm, transient pulmonary edema MRC dyspnoea scale 1- Not troubled by breathlessness except on strenuous exercise 2 - Short of breath when hurrying or walking up a slight hill 3 - Walks slower than contemporaries on level ground because of breathlessness or has to stop for breath when walking at own pace 4 - Stops for breath after walking about 100 m or after a few minutes on level ground 5 - Too breathless to leave the house, or breathless when dressing or undressing. The New York heart Association functional and therapeutic classification applied to dyspnea Grade 1 : No breathlessness Grade 2 :Breathlessness on severe exertion Grade 3 :Breathlessness on mild exertion Grade 4 : Breathlessness at rest cough • productive: bronchiectasis, bronchitis, abscess, bacterial pneumonia, TB • nonproductive: viral infections, interstitial lung disease, anxiety, allergy • wheezy: suggests bronchospasm, asthma, allergy • nocturnal: asthma, CHF, postnasal drip, GERD, or aspiration • barking: epiglottal disease (croup) in children • positional: abscess, tumour sputum • mucoid: asthma, tumour, TB, emphysema • purulent green: -

US-90314-Quiz-Respiratory.Pdf
Respiratory 14Mar2009 Respiratory #1 – Histology 1) Which of the following belongs to the respiratory portion of the air passage, not the conduction portion? a) Bronchioles b) Bronchi c) Trachea d) Larynx e) Pharynx 2.1) Which of the following respiratory cell types create mucus? a) Brush cells b) Basal cells c) Ciliated cells d) Olfactory cells e) Goblet cells 2.2) What type of cells line the vestibular chamber of the nasal cavity? a) Bipolar olfactory neurons b) Pseudostratified columnar c) Ciliated tall columnar d) Stratified squamous e) Small granular cells 3) What type of epithelial cell characterizes the larynx and respiratory tract? a) Unciliated pseudostratified squamous b) Ciliated pseudostratified squamous c) Unciliated pseudostratified columnar d) Ciliated pseudostratified columnar e) Brush cells and goblet cells 4.1) What type of tracheal cells function as receptor cells as their basal surface is in synaptic contact with afferent nerve endings? a) Ciliated cells b) Mucous cells c) Brush cells d) Small granule cells e) Basal cells 4.2) The C-shaped cartilaginous layer is a unique feature of which of the following? a) Bronchioles b) Bronchi c) Trachea d) Larynx e) Pharynx 5) Disappearance of what histological layer signifies a change from the bronchi to the bronchioles? a) Mucosa b) Muscularis c) Submucousal d) Cartilage plates e) Adventitia DO NOT DISTRIBUTE - 1 - Respiratory 14Mar2009 6) Which of the following best describes the epithelial layer of the small bronchioles? a) Simple cuboid epithelium with Clara cells b) Pseudostratified -
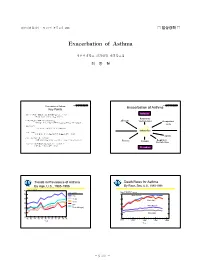
Exacerbation of Asthma
대한내과학회지 : 제73권 부록 2 호 2007 □ 임상강좌 □ Exacerbation of Asthma 성균관대학교 의과대학 내과학교실 최 동 철 2007년내과학회임상강좌 2007년내과학회임상강좌 Exacerbation of Asthma Exacerbation of Asthma Key Points -천식의 악화란 호흡곤란, 기침, 천명,흉부 압박감 등의 증상 중 Inducers 한가지 이상이 나타나는 것을 가리킨다. Respiratory -천식이 악화되면 숨을 내쉬기 어려워 지고 Allergens viral infections Occupational 폐기능검사에서 최대호기유속이나 1초간 노력성 호기량이 감소한다. agents -주요한 치료는 기관지 확장제, 스테로이드 및 산소 투여이다. Asthma flare -치료 목표는 환기 장애와 저산소증을 개선하고 재발을 방지하는 것이다. Aspirin -심한 천식 발작은 응급 상황이며 적절한 시설이 있는 곳에서 치료해야 하고 전문가의 감독이 필요하다. Exercise Irritants Respiratory viral infections -가벼운천식발작(최대호기유속의 감소가 20% 미만)은 지역의료기관에서 치료할 수 있다 Provokers Trends in Prevalence of Asthma Death Rates for Asthma By Age, U.S., 1985-1996 By Race, Sex, U.S., 1980-1998 80 Rate/1,000 Persons Age (years) Rate/100,000 Persons 70 5 <18 Black Female 60 18-44 4 Black Male 45-64 50 65+ 3 White Female 40 Total (All Ages) 2 30 White Male 1 20 85 86 87 88 89 90 91 92 93 94 95 96 0 1980 Year 1985 1990 1995 2000 Year - S 737 - - 대한내과학회지 : 제73권 부록 2 호 2007 - 2007년내과학회임상강좌 2007년내과학회임상강좌 Exacerbation of Asthma Histrory (1) A. Timing of dyspnea Acute onset Insiduous onset anxiety, hyperventilation COPD Q1: 환자의 호흡곤란이 천식 발작인가? asthma, pulmonary edema interstitial fibrosis pulmonary embolism, sarcoidosis chest trauma diseases of chest wall (pneumothorax, rib fracture, or diaphragm contusion) spontaneous pneumothorax 2007년내과학회임상강좌 2007년내과학회임상강좌 Exacerbation of Asthma Exacerbation of Asthma Histrory (2) Histrory (3) B. Relationship to physical activity Nocturnal dyspnea ATS Shortness of Breath scale Asthma, CHF,