Analysis of Orbital Decay Time for the Classical Hydrogen Atom Interacting with Circularly Polarized Electromagnetic Radiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PDF (03Ragozzine Exo-Interiors.Pdf)
20 Chapter 2 Probing the Interiors of Very Hot Jupiters Using Transit Light Curves This chapter will be published in its entirety under the same title by authors D. Ragozzine and A. S. Wolf in the Astrophysical Journal, 2009. Reproduced by permission of the American Astro- nomical Society. 21 Abstract Accurately understanding the interior structure of extra-solar planets is critical for inferring their formation and evolution. The internal density distribution of a planet has a direct effect on the star-planet orbit through the gravitational quadrupole field created by the rotational and tidal bulges. These quadrupoles induce apsidal precession that is proportional to the planetary Love number (k2p, twice the apsidal motion constant), a bulk physical characteristic of the planet that depends on the internal density distribution, including the presence or absence of a massive solid core. We find that the quadrupole of the planetary tidal bulge is the dominant source of apsidal precession for very hot Jupiters (a . 0:025 AU), exceeding the effects of general relativity and the stellar quadrupole by more than an order of magnitude. For the shortest-period planets, the planetary interior induces precession of a few degrees per year. By investigating the full photometric signal of apsidal precession, we find that changes in transit shapes are much more important than transit timing variations. With its long baseline of ultra-precise photometry, the space-based Kepler mission can realistically detect apsidal precession with the accuracy necessary to infer the presence or absence of a massive core in very hot Jupiters with orbital eccentricities as low as e ' 0:003. -

Orbital Lifetime Predictions
Orbital LIFETIME PREDICTIONS An ASSESSMENT OF model-based BALLISTIC COEFfiCIENT ESTIMATIONS AND ADJUSTMENT FOR TEMPORAL DRAG co- EFfiCIENT VARIATIONS M.R. HaneVEER MSc Thesis Aerospace Engineering Orbital lifetime predictions An assessment of model-based ballistic coecient estimations and adjustment for temporal drag coecient variations by M.R. Haneveer to obtain the degree of Master of Science at the Delft University of Technology, to be defended publicly on Thursday June 1, 2017 at 14:00 PM. Student number: 4077334 Project duration: September 1, 2016 – June 1, 2017 Thesis committee: Dr. ir. E. N. Doornbos, TU Delft, supervisor Dr. ir. E. J. O. Schrama, TU Delft ir. K. J. Cowan MBA TU Delft An electronic version of this thesis is available at http://repository.tudelft.nl/. Summary Objects in Low Earth Orbit (LEO) experience low levels of drag due to the interaction with the outer layers of Earth’s atmosphere. The atmospheric drag reduces the velocity of the object, resulting in a gradual decrease in altitude. With each decayed kilometer the object enters denser portions of the atmosphere accelerating the orbit decay until eventually the object cannot sustain a stable orbit anymore and either crashes onto Earth’s surface or burns up in its atmosphere. The capability of predicting the time an object stays in orbit, whether that object is space junk or a satellite, allows for an estimate of its orbital lifetime - an estimate satellite op- erators work with to schedule science missions and commercial services, as well as use to prove compliance with international agreements stating no passively controlled object is to orbit in LEO longer than 25 years. -

NASA Process for Limiting Orbital Debris
NASA-HANDBOOK NASA HANDBOOK 8719.14 National Aeronautics and Space Administration Approved: 2008-07-30 Washington, DC 20546 Expiration Date: 2013-07-30 HANDBOOK FOR LIMITING ORBITAL DEBRIS Measurement System Identification: Metric APPROVED FOR PUBLIC RELEASE – DISTRIBUTION IS UNLIMITED NASA-Handbook 8719.14 This page intentionally left blank. Page 2 of 174 NASA-Handbook 8719.14 DOCUMENT HISTORY LOG Status Document Approval Date Description Revision Baseline 2008-07-30 Initial Release Page 3 of 174 NASA-Handbook 8719.14 This page intentionally left blank. Page 4 of 174 NASA-Handbook 8719.14 This page intentionally left blank. Page 6 of 174 NASA-Handbook 8719.14 TABLE OF CONTENTS 1 SCOPE...........................................................................................................................13 1.1 Purpose................................................................................................................................ 13 1.2 Applicability ....................................................................................................................... 13 2 APPLICABLE AND REFERENCE DOCUMENTS................................................14 3 ACRONYMS AND DEFINITIONS ...........................................................................15 3.1 Acronyms............................................................................................................................ 15 3.2 Definitions ......................................................................................................................... -

NEAR of the 21 Lunar Landings, 19—All of the U.S
Copyrights Prof Marko Popovic 2021 NEAR Of the 21 lunar landings, 19—all of the U.S. and Russian landings—occurred between 1966 and 1976. Then humanity took a 37-year break from landing on the moon before China achieved its first lunar touchdown in 2013. Take a look at the first 21 successful lunar landings on this interactive map https://www.smithsonianmag.com/science-nature/interactive-map-shows-all-21-successful-moon-landings-180972687/ Moon 1 The near side of the Moon with the major maria (singular mare, vocalized mar-ray) and lunar craters identified. Maria means "seas" in Latin. The maria are basaltic lava plains: i.e., the frozen seas of lava from lava flows. The maria cover ∼ 16% (30%) of the lunar surface (near side). Light areas are Lunar Highlands exhibiting more impact craters than Maria. The far side is pocked by ancient craters, mountains and rugged terrain, largely devoid of the smooth maria we see on the near side. The Lunar Reconnaissance Orbiter Moon 2 is a NASA robotic spacecraft currently orbiting the Moon in an eccentric polar mapping orbit. LRO data is essential for planning NASA's future human and robotic missions to the Moon. Launch date: June 18, 2009 Orbital period: 2 hours Orbit height: 31 mi Speed on orbit: 0.9942 miles/s Cost: 504 million USD (2009) The Moon is covered with a gently rolling layer of powdery soil with scattered rocks called the regolith; it is made from debris blasted out of the Lunar craters by the meteor impacts that created them. -

Study of Extraterrestrial Disposal of Radioactive Wastes
NASA TECHNICAL NASA TM X-71557 MEMORANDUM N--- XI NASA-TM-X-71557) STUDY OF N74-22776 EXTRATERRESTRIAL DISPOSAL OF READIOACTIVE WASTES. PART 1: SPACE TRANSPORTATION. AN.. DESTINATION CONSIDERATIONS. FOR, (NASA) Unclas 64 p HC $6.25 CSCL 18G G3/05 38494 STUDY OF EXTRATERRESTRIAL DISPOSAL OF RADIOACTIVE WASTES by R. L. Thompson, J. R. Ramler, and S. M. Stevenson Lewis Research Center Cleveland, Ohio 44135 May 1974 This information is being published in prelimi- nary form in order to expedite its early release. I STUDY OF EXTRATERRESTRIAL DISPOSAL OF RADIOACTIVE WASTES Part I Space Transportation and Destination Considerations for Extraterrestrial Disposal of Radioactive W4astes by R. L. Thompson, J. R. Ramler, and S. M. Stevenson Lewis Research Center CT% CSUMMARY I NASA has been requested by the AEC to conduct a feasibility study of extraterrestrial (space) disposal of radioactive waste. This report covers the initial work done on only one part of the NASA study, the evaluation and comparison of possible space destinations and space transportation systems. Only current or planned space transportation systems have been considered thus far. The currently planned Space Shuttle was found to be more cost-effective than current expendable launch vehicles by about a factor of 2. The Space Shuttle requires a third stage to perform the waste disposal missions. Depending on the particular mission, this third stage could be either a reusable space tug or an expendable stage such as a Centaur. Of the destinations considered, high Earth orbits (between geostationary and lunar orbit altitudes), solar orbits (such as a 0.90 AU circular solar orbit) or a direct injection to solar system escape appear to be the best candidates. -
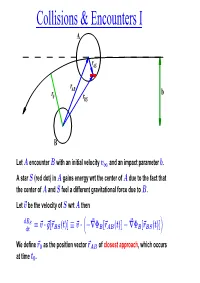
Collisions & Encounters I
Collisions & Encounters I A rAS ¡ r r AB b 0 rBS B Let A encounter B with an initial velocity v and an impact parameter b. 1 A star S (red dot) in A gains energy wrt the center of A due to the fact that the center of A and S feel a different gravitational force due to B. Let ~v be the velocity of S wrt A then dES = ~v ~g[~r (t)] ~v ~ Φ [~r (t)] ~ Φ [~r (t)] dt · BS ≡ · −r B AB − r B BS We define ~r0 as the position vector ~rAB of closest approach, which occurs at time t0. Collisions & Encounters II If we increase v then ~r0 b and the energy increase 1 j j ! t0 ∆ES(t0) ~v ~g[~rBS(t)] dt ≡ 0 · R dimishes, simply because t0 becomes smaller. Thus, for a larger impact velocity v the star S withdraws less energy from the relative orbit between 1 A and B. This implies that we can define a critical velocity vcrit, such that for v > vcrit galaxy A reaches ~r0 with sufficient energy to escape to infinity. 1 If, on the other hand, v < vcrit then systems A and B will merge. 1 If v vcrit then we can use the impulse approximation to analytically 1 calculate the effect of the encounter. < In most cases of astrophysical interest, however, v vcrit and we have 1 to resort to numerical simulations to compute the outcome∼ of the encounter. However, in the special case where MA MB or MA MB we can describe the evolution with dynamical friction , for which analytical estimates are available. -

Orbital Mechanics Joe Spier, K6WAO – AMSAT Director for Education ARRL 100Th Centennial Educational Forum 1 History
Orbital Mechanics Joe Spier, K6WAO – AMSAT Director for Education ARRL 100th Centennial Educational Forum 1 History Astrology » Pseudoscience based on several systems of divination based on the premise that there is a relationship between astronomical phenomena and events in the human world. » Many cultures have attached importance to astronomical events, and the Indians, Chinese, and Mayans developed elaborate systems for predicting terrestrial events from celestial observations. » In the West, astrology most often consists of a system of horoscopes purporting to explain aspects of a person's personality and predict future events in their life based on the positions of the sun, moon, and other celestial objects at the time of their birth. » The majority of professional astrologers rely on such systems. 2 History Astronomy » Astronomy is a natural science which is the study of celestial objects (such as stars, galaxies, planets, moons, and nebulae), the physics, chemistry, and evolution of such objects, and phenomena that originate outside the atmosphere of Earth, including supernovae explosions, gamma ray bursts, and cosmic microwave background radiation. » Astronomy is one of the oldest sciences. » Prehistoric cultures have left astronomical artifacts such as the Egyptian monuments and Nubian monuments, and early civilizations such as the Babylonians, Greeks, Chinese, Indians, Iranians and Maya performed methodical observations of the night sky. » The invention of the telescope was required before astronomy was able to develop into a modern science. » Historically, astronomy has included disciplines as diverse as astrometry, celestial navigation, observational astronomy and the making of calendars, but professional astronomy is nowadays often considered to be synonymous with astrophysics. -

Orbital Decay Analysis for Debris Deorbiting Cubesats in Leo: a Case Study for the Velox-Ii Deorbit Mission
ORBITAL DECAY ANALYSIS FOR DEBRIS DEORBITING CUBESATS IN LEO: A CASE STUDY FOR THE VELOX-II DEORBIT MISSION Sarat Chandra Nagavarapu(1), Amal Chandran(1), and Daniel E. Hastings(2) (1)Satellite Research Centre (SaRC), Nanyang Technological University, Singapore, 639798, Email: [email protected], [email protected] (2)Aeronautics & Astronautics Department, MIT, Cambridge, MA, USA, 02139, Email: [email protected] ABSTRACT Keywords: Space debris; LEO; orbital decay; debris re- moval; VELOX-II; CubeSat. Since the launch of Sputnik 1 into space in 1957, hu- mans have sent thousands of rockets and more than ten thousand satellites into Earth’s orbit. With hundreds of 1. INTRODUCTION more launched every year, in-space collisions and the creation of high-speed debris fragments are becoming in- creasingly likely, threatening future space missions. The Space debris is any man-made object in orbit about the term orbital debris comprises defunct satellites, spent up- Earth that no longer serves a useful function. The Earth per stages, fragments resulted from collisions, etc., which orbit is in serious danger caused by millions of these pose a significant threat to many other operational satel- space debris. Since the beginning of the space age, more lites in their vicinity. With the advent of satellite mega- than 10,680 satellites have been placed into orbit [1]. Out constellations in low Earth orbit (LEO), the LEO envi- of which 6,250 are still in the orbit and only 3,700 are op- ronment is becoming extremely crowded. In addition to erational, leaving an astonishing 2550 dead satellites in the government space organizations, several private space the orbit. -

Atmospheric Drag Effects on Modelled LEO Satellites During the July 2000 Bastille Day Event in Contrast to an Interval of Geomagnetically Quiet Conditions Victor U
https://doi.org/10.5194/angeo-2020-33 Preprint. Discussion started: 29 June 2020 c Author(s) 2020. CC BY 4.0 License. Atmospheric drag effects on modelled LEO satellites during the July 2000 Bastille Day event in contrast to an interval of geomagnetically quiet conditions Victor U. J. Nwankwo1, William Denig2, Sandip K. Chakrabarti3, Muyiwa P. Ajakaiye1, Johnson Fatokun1, Adeniyi W. Akanni1, Jean-Pierre Raulin4, Emilia Correia4, and John E. Enoh5 1Anchor University, Lagos 100278, Nigeria 2St. Joseph College of Maine, Standish, ME 04084, U.S.A 3Indian Centre for Space Physics, Kolkata 700084, India 4Centro de Rádio Astronomia e Astrofísica Mackenzie, Universidade Presbiteriana Mackenzie, São Paulo, Brazil 5Interorbital systems, Mojave, CA 93502-0662, U.S.A. Correspondence: Victor U. J. Nwankwo ([email protected]) Abstract. In this work we simulated the effects of atmospheric drag on two model SmallSats in Low Earth Orbit (LEO) with different ballistic coefficients during 1-month intervals of solar-geomagnetic quiet and perturbed conditions. The goal of this effort was to quantify how solar-geomagnetic activity influences atmospheric drag and perturbs satellite orbits. Atmospheric drag compromises satellite operations due to increased ephemeris errors, attitude positional uncertainties and premature satel- 5 lite re-entry. During a 1-month interval of generally quiescent solar-geomagnetic activity (July 2006) the decay in altitude (h) 3 2 3 was a modest 0.53 km (0.66 km) for the satellite with the smaller (larger) ballistic coefficient of 2.2 10− m /kg (3.03 10− × × m2/kg). The associated Orbital Decay Rates (ODRs) during this quiet interval ranged from 13 m/day to 23 m/day (from 16 m/day to 29 m/day). -

Kickstart Physics Space
KICKSTART PHYSICS SPACE 1. SPEED AND ESCAPE VELOCITY 2. PROJECTILE MOTION 3. ACCELERATION AND G-FORCES 4. ‘C’ AND RELATIVITY 5. EINSTEIN Kickstart would like to acknowledge and pay respect to the traditional owners of the land – the Gadigal people of the Eora Nation. It is upon their ancestral lands that the University of Sydney is built. As we share our knowledge, teaching, learning, and research practices within this University may we also pay respect to the knowledge embedded forever within the Aboriginal Custodianship of Country. For more information head to http://sydney.edu.au/science/outreach/high-school/kickstart/index.shtml The University of Sydney School of Physics Space Get to Know the Inner Space Risk Analysis Conduct a risk analysis by filling out the following table. List 3 risks to do with this investigation in the 2nd year lab. Also, list 3 risks in an industry where physics is used. − What are the consequences of those risks? Use the risk matrix below to make your judgment. − What precautions would you take to stop those risks from coming about? − What steps would you take to mitigate those risks? Risk Consequence Precaution/Mitigation Electricity Electrocution (High) Safety Switch Radiation Radiation Sickness (High) Low Dose/Shielding Kickstart Equipment Tripping and Injury (Medium) Don’t Touch Water Slipping and Injury (Medium) No Food/Drink in Lab LN2 Frostbite (High) Personal Protective Equipment Industry – Finance/Tech, Games, Solar Power, Medical Physics, Radiology Physics Research – University, See above, jobs might require See above, jobs will require Industry Academia more specific considerations more specific considerations Public Service – CSIRO, DSTO, NMI, Questacon, Government, Teachers What sort of careers do you think you could get if you studied this topic at the University of Sydney? 1 The University of Sydney School of Physics Space Speed & Escape Velocity There are a number of different types of orbits in orbital systems. -

Samuel YW Low (Samuel [email protected])
Assessment of Orbit Maintenance Strategies for Small Satellites Samuel Y. W. Low ([email protected]), Singapore University of Technology and Design | Y. X. Chia ([email protected]), DSO National Laboratories Objectives: To explore and validate how various orbit maintenance strategies for small satellites can be optimized for V usage, by varying the tolerance bands as the independent variable. Δ Needs: A computationally efficient and accurate model for the rate of orbit decay, and a model small satellite in LEO as test parameters. Summary: Three methods were proposed: one of forced-Keplerian orbits using constant thrust, one via Hohmann transfers, and one via cyclical direct burns. Results show potential for minimizing V usage, and also reveal and interesting connection as tolerance bands tend to infinitesimally small values. 0. THE ORBITAL DECAY MODEL Δ II. METHOD OF HOHMANN TRANSFERS III. METHOD OF CYCLICAL DIRECT BURNS VELOX-CI Satellite Information Decay Data 2.444 Payload: Radio Occultation Unit from Model km 615 X 608 X 848 mm3 Decay Data 2.566 L (km) Mass = 123 kg from GPS km R (km) Semi major axis = 6928.14 km Fig 2: Decay data comparisons Altitude = 545 km with proposed model Inclination = 15 degrees Fig 1: Model satellite and orbit parameters used in the simulation RAAN, ARP, True Anomaly = 0 Model Satellite & Parameters: VELOX-CI Fig 4: Hohmann transfer, Orbit Characteristics: Circular orbit, near-equatorial against radial axis tolerance band (km) Fig 6: Single ∆V of single the in-track axis tolerance band (km) Orbit Decay Validation Period: 16/12/2015 to 11/11/2016 . -

Rapid Eccentricity Oscillations and the Mergers of Compact Objects In
Mon. Not. R. Astron. Soc. 000, 000–000 (0000) Printed 29 June 2018 (MN LATEX style file v2.2) Rapid Eccentricity Oscillations and the Mergers of Compact Objects in Hierarchical Triples Joe M. Antognini1, Benjamin J. Shappee1, Todd A. Thompson1,2, Pau Amaro-Seoane3 1 Department of Astronomy, The Ohio State University, Columbus, Ohio 43210, USA 2 Center of Cosmology and Astro-Particle Physics, The Ohio State University, Columbus, Ohio 43210, USA 3 Max Planck Institut f¨ur Gravitationsphysik (Albert-Einstein Institut), D-14476 Potsdam, Germany E-mail: [email protected] 29 June 2018 ABSTRACT Kozai-Lidov (KL) oscillations can accelerate compact object mergers via gravita- tional wave (GW) radiation by driving the inner binaries of hierarchical triples to high eccentricities. We perform direct three-body integrations of high mass ratio compact object triple systems using Fewbody including post-Newtonian terms. We find that the inner binary undergoes rapid eccentricity oscillations (REOs) on the timescale of the outer orbital period which drive it to higher eccentricities than secular theory would otherwise predict, resulting in substantially reduced merger times. For a uniform distribution of tertiary eccentricity (e2), ∼ 40% of systems merge within ∼ 1 − 2 ec- centric KL timescales whereas secular theory predicts that only ∼20% of such systems merge that rapidly. This discrepancy becomes especially pronounced at low e2, with secular theory overpredicting the merger time by many orders of magnitude. We show that a non-negligible fraction of systems have eccentricity > 0.8 when they merge, in contrast to predictions from secular theory. Our results are applicable to high mass ratio triple systems containing black holes or neutron stars.