Current and Future Development in Lung Cancer Diagnosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genetic Markers in Lung Cancer Diagnosis: a Review
International Journal of Molecular Sciences Review Genetic Markers in Lung Cancer Diagnosis: A Review Katarzyna Wadowska 1 , Iwona Bil-Lula 1 , Łukasz Trembecki 2,3 and Mariola Sliwi´ ´nska-Mosso´n 1,* 1 Department of Medical Laboratory Diagnostics, Division of Clinical Chemistry and Laboratory Haematology, Wroclaw Medical University, 50-556 Wroclaw, Poland; [email protected] (K.W.); [email protected] (I.B.-L.) 2 Department of Radiation Oncology, Lower Silesian Oncology Center, 53-413 Wroclaw, Poland; [email protected] 3 Department of Oncology, Faculty of Medicine, Wroclaw Medical University, 53-413 Wroclaw, Poland * Correspondence: [email protected]; Tel.: +48-71-784-06-30 Received: 1 June 2020; Accepted: 25 June 2020; Published: 27 June 2020 Abstract: Lung cancer is the most often diagnosed cancer in the world and the most frequent cause of cancer death. The prognosis for lung cancer is relatively poor and 75% of patients are diagnosed at its advanced stage. The currently used diagnostic tools are not sensitive enough and do not enable diagnosis at the early stage of the disease. Therefore, searching for new methods of early and accurate diagnosis of lung cancer is crucial for its effective treatment. Lung cancer is the result of multistage carcinogenesis with gradually increasing genetic and epigenetic changes. Screening for the characteristic genetic markers could enable the diagnosis of lung cancer at its early stage. The aim of this review was the summarization of both the preclinical and clinical approaches in the genetic diagnostics of lung cancer. The advancement of molecular strategies and analytic platforms makes it possible to analyze the genome changes leading to cancer development—i.e., the potential biomarkers of lung cancer. -

Invasive Cervical Cancer Audit; EU Guidelines for Quality Assurance
The 4th EFCS Annual Tutorial Ospedale Universitario di Cattinara, Strada di Fiume, Trieste Handouts for lectures and workshops – I I - Gynaecological cytopathology Mrs Rietje Salet‐van‐de Pol, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands Gynecological cytology: technical aspects ............................................................................... 2 Non‐neoplastic gynecological cytology .................................................................................... 6 • Dr Giovanni Negri, General Hospital of Bolzano, Bozano SIL and cancer; ASC‐US, ASC‐H, diagnostic pitfalls and look‐alikes; glandular abnormalities 11 • Dr Amanda Herbert, Guy’s & St Thomas’ NHS Foundation Trust, London Invasive cervical cancer audit; EU guidelines for quality assurance ...................................... 17 1 Gynecological cytology: technical aspects Rietje Salet-van de Pol Important in specimen processing is to obtain as much as possible well preserved cells for microscopically evaluation. The quality of the smear depends on cell sampling, fixation and staining. For obtaining enough cervical material you are dependent on the cell sampler. For cervical cytology two types of specimen are available: conventional smears and liquid based cytology (LBC). Conventional, Thinprep and Surepath slides In conventional cytology the cell sampler makes the smear and is responsible for the fixation of the cells. Reasons for unsatisfactory conventional smears can be obscuring blood or inflammatory cells, thick smears with overlapping cells, poor preservation of the cells due to late fixation and low cellularity. In LBC the cell sampler immediately transferred the cellular material into a vial with fixative (fixating solution) which gives a better preservation of the cells. The laboratory is responsible for processing of the smear. LBC gives equally distribution of the cells in a thin cell layer of well preserved cells. The rate of unsatisfactory smears is lower. -
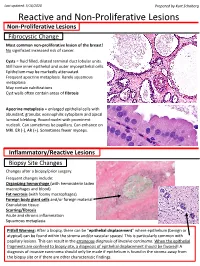
Reactive and Non-Proliferative Lesions
Last updated: 5/16/2020 Prepared by Kurt Schaberg Reactive and Non-Proliferative Lesions Non-Proliferative Lesions Fibrocystic Change Most common non-proliferative lesion of the breast! No significant increased risk of cancer. Cysts = fluid filled, dilated terminal duct lobular units. Still have inner epithelial and outer myoepithelial cells. Epithelium may be markedly attenuated. Frequent apocrine metaplasia. Rarely squamous metaplasia May contain calcifications Cyst walls often contain areas of fibrosis Apocrine metaplasia = enlarged epithelial cells with abundant, granular, eosinophilic cytoplasm and apical luminal blebbing. Round nuclei with prominent nucleoli. Can sometimes be papillary. Can enhance on MRI. ER (-), AR (+). Sometimes fewer myoeps. Inflammatory/Reactive Lesions Biopsy Site Changes Changes after a biopsy/prior surgery. Frequent changes include: Organizing hemorrhage (with hemosiderin laden macrophages and blood) Fat necrosis (with foamy macrophages) Foreign body giant cells and/or foreign material Granulation tissue Scarring/fibrosis Acute and chronic inflammation Squamous metaplasia Pitfall Warning: After a biopsy, there can be “epithelial displacement” where epithelium (benign or atypical) can be found within the stroma and/or vascular spaces! This is particularly common with papillary lesions. This can result in the erroneous diagnosis of invasive carcinoma. When the epithelial fragments are confined to biopsy site, a diagnosis of epithelial displacement should be favored! A diagnosis of invasive carcinoma should -

Primary Immature Teratoma of the Thigh Fig
CORRESPONDENCE 755 8. Gray W, Kocjan G. Diagnostic Cytopathology. 2nd ed. London: Delete all that do not apply: Elsevier Health Sciences, 2003; 677. 9. Richards A, Dalrymple C. Abnormal cervicovaginal cytology, unsatis- Cervix, colposcopic biopsy/LLETZ/cone biopsy: factory colposcopy and the use of vaginal estrogen cream: an obser- vational study of clinical outcomes for women in low estrogen states. Diagnosis: NIL (No intraepithelial lesion WHO 2014) J Obstet Gynaecol Res 2015; 41: 440e4. LSIL (CIN 1 with HPV effect WHO 2014) 10. Darragh TM, Colgan TJ, Cox T, et al. The lower anogenital squamous HSIL (CIN2/3 WHO 2014) terminology standardization project for HPV-associated lesions: back- Squamous cell carcinoma ground and consensus recommendation from the College of American Immature squamous metaplasia Pathologists and the American Society for Colposcopy and Cervical Adenocarcinoma in situ (AIS, HGGA) e Adenocarcinoma Pathology. Arch Pathol Lab Med 2012; 136: 1267 97. Atrophic change 11. McCluggage WG. Endocervical glandular lesions: controversial aspects e Extending into crypts: Not / Idenfied and ancillary techniques. J Clin Pathol 2013; 56: 164 73. Epithelial stripping: Not / Present 12. World Health Organization (WHO). Comprehensive Cervical Cancer Invasive disease: Not / Idenfied / Micro-invasive Control: A Guide to Essential Practice. 2nd ed. Geneva: WHO, 2014. Depth of invasion: mm Transformaon zone: Not / Represented Margins: DOI: https://doi.org/10.1016/j.pathol.2019.07.014 Ectocervical: Not / Clear Endocervical: Not / Clear Circumferenal: Not / Clear p16 status: Negave / Posive Primary immature teratoma of the thigh Fig. 3 A proposed synoptic reporting format for pathologists reporting colposcopic biopsies and cone biopsies or LLETZ. Sir, Teratomas are germ cell tumours composed of a variety of HSIL, AIS, micro-invasive or more advanced invasive dis- somatic tissues derived from more than one germ layer 12 ease. -

Section Ii: General Abstracting Instructions
SECTION II: GENERAL ABSTRACTING INSTRUCTIONS 60 SECTION II: GENERAL ABSTRACTING INSTRUCTIONS It is the responsibility of every abstractor to know the content of the FCDS Data Acquisition Manual (DAM) and to update it upon receipt of any change from FCDS. Should you need training in cancer registry data collection, please visit the FCDS Learning Management System and consider taking the FCDS Abstracting Basics Course to gain a better understanding of the skills and training required to meet FCDS abstracting requirements and the national standards used when abstracting and coding cancer cases. This manual is intended to explain in detail each data item required for Florida Cancer Data System (FCDS) case reporting. It should be used as the primary information resource for any data item that must be coded and documented in accordance with Florida cancer reporting rules and statutes. Descriptions are only intended to provide sufficient detail to achieve consensus in submitting the required data. In no way does this manual imply any restriction on the type or degree of detail information collected, classified or studied within any healthcare facility-based cancer registry. Special Use Fields are available as needed. Basic Rules: 1) Always refer to the FCDS Data Acquisition Manual when completing an abstract. 2) Always submit a separate abstract for each reportable primary neoplasm identified. 3) Use leading zeros when necessary to right justify. 4) Text is required to adequately justify ALL coded values and to document supplemental information such as patient and family history of malignancy. Data items MUST be well documented in text field(s); specifically, Place of Diagnosis, Physical Exam, X-rays and Scans, Scopes and Diagnostic Tools, Surgical Procedures and Findings, Laboratory and Pathology (including: Dates of Specimen Collection, Primary Site, Histology, Behavior and Grade), and the Collaborative Stage data items including both core items and site specific factors. -

Cancer Basics for the Caregiver It Is Common to Make Many Assumptions When You Hear the Word “Cancer.” Cancer Is Not One Disease, but Rather a Family of Diseases
Caregiver’s Guide Types of Caregiving Caregiving can range from 24/7 hands-on assistance to driving someone to appointments to long-distance caregiving. Every situation is different. Your loved one has cancer and you want to help. At first, it all seems overwhelming. Everything that you took for granted is suddenly uncertain. Many caregivers are naturally worried about the person with cancer, and also worried about the rest of life—taking care of other family members, paying the bills, maintaining the house, and so much more. It’s important to realize two things: 1) You’re not alone— many other people have been in this situation before, and 2) there are resources available to help. We’ve prepared this booklet to guide and assist you. Much depends on the needs of the patient, your What’s essential is to understand that the role of the relationship with the patient, and practical matters loved one is to support and comfort, not to “fix” the such as where you live. problem. Every caregiving situation has the potential to be both When people are diagnosed with cancer, they don’t rewarding and stressful—often at the same time. want their loved ones to say, “I promise you that you’ll be cured.” In addition to worrying about your loved one’s cancer, you may be running the household, struggling What they want to hear is, “I love you and I’ll be here with piles of incomprehensible insurance forms, with you for whatever comes.” communicating with far-flung family members, and trying to earn enough money to pay the mounting bills. -

Welcome to the Cancer Resource Center!
Welcome to the Cancer Resource Center! We understand that this is a difficult time for you and your family. We are here to offer assistance throughout your diagnosis, treatment, recovery, and beyond. The welcome folder describes some of the services and support we provide to individuals and families affected by cancer. Please don’t hesitate to contact us if you have questions about any information contained in this folder. Our staff is happy to talk with you one-on-one to answer questions and to provide information and resources available both locally and nationally. We meet with couples and families as well and we always respect the confidentiality of everyone we meet with. We share information only when given permission to do so. CRC has a lending library of books and other materials that covers a wide range of cancer-related topics as well as a boutique featuring free wigs, hats, scarves, and other items that can be useful during some types of treatment. Our many support groups for individuals with cancer and their loved ones play an important role in providing assistance and connection to others with similar experiences. Our Financial Advocacy program can help provide assistance with financial concerns if needed. Our website (www.crcfl.net) includes many additional resources that may be of assistance to you and your family. We encourage you to visit it. If you do not have a computer, we will be happy to assist you in finding cancer-related information that we can mail to you. Our staff and volunteers are here to help you in any way we can. -

1 2 3 4 5 6 7 8 9 10 11 12 13 1 Presidential Advisory Committee
Presidential Advisory Committee 1 Department of Health and Human Services Centers for Disease Control and Prevention (CDC) National Institute for Occupational Safety and Health 1 (NIOSH) Advisory Board on Radiation and Worker Health 2 3 4 VOLUME I 5 6 7 The verbatim transcript of the Meeting of the Advisory Board on Radiation and Worker Health 8 held at the Washington Court Hotel, 525 New Jersey Avenue, N.W., Washington, D.C., on May 2 and 3, 9 2002. 10 NANCY LEE & ASSOCIATES Certified Verbatim Reporters P.O. Box 451196 11 Atlanta, Georgia 31145-9196 (404) 315-8305 12 13 C O N T E N T S 2 Vol. I Registration and Welcome Dr. Paul Ziemer, Chair 1 Mr. Larry Elliott, Executive Secretary. .8 Welcome and Opening Remarks Dr. Kathleen Rest, NIOSH . .11 2 Review and Approval of Draft Minutes Dr. Paul Ziemer, Chair. 18 3 Program Status Report Mr. Larry Elliott, Executive Secretary . .36 Changes to Probability of Causation Rule 4 (42 CFR Part 82) Mr. Ted Katz, NIOSH . 70 NCI-IREP 5 Dr. Charles Land, NCI . 82 NIOSH-IREP in use by DOL Dr. Mary Schubauer-Berigan, NIOSH . .115 6 Mr. Russ Henshaw, NIOSH . .176 Topics for Future Discussion Dr. Paul Ziemer, Chair . 193 7 Public Comment . 207 Discussion of Changes in the Rule . .216 8 Adjourn . .223 9 10 11 12 13 C O N T E N T S 3 Vol. II Registration and Welcome Dr. Paul Ziemer, Chair Mr. Larry Elliott, Executive Secretary . 227 1 Administrative Housekeeping Ms. Cori Homer, NIOSH . .227 2 Discussion of Rules. -

Squamous Metaplasia of the Tracheal Epithelium in Children
Thorax: first published as 10.1136/thx.31.2.167 on 1 April 1976. Downloaded from Thorax (1976), 31, 167. Squamous metaplasia of the tracheal epithelium in children AVINASH MITHAL' and JOHN L. EMERY2 The Chest Clinic, Lincoln' and The Children's Hospital, Sheffield' Mithal, A. and Emery, J. L. (1976). Thorax, 31, 167-171. Squamous metaplasia of the tracheal epithelium in children. Thirty-seven (16%) tracheas from 2170 children showed squamous metaplasia. (Cases with tracheo-oesophageal fistula and congenital heart disease were excluded.) The metaplasia extended into the bronchi in 15 cases. Features of pulmonary retention were present in seven cases. Respiratory infection, probably viral, seemed to be the most significant causative factor in 20 children, including those with cystic fibrosis. Tracheal instrumentation was a possible factor in 11 cases but oxygen therapy alone did not seem important. The metaplasia was almost certainly congenital in one child and probably in two others but no stillborn infants showed metaplasia. In many children the metaplasia seemed to be due to a combination of factors. Squamous metaplasia of the trachea in childhood Tracheas from children with tracheo-oesophageal has been described in cases of measles (Gold- fistula and those with congenital heart disease or zieher, 1918), influenza (Askanazy, 1919), cystic other gross deformities were excluded. There were fibrosis of the pancreas (Zuelzer and Newton, thus 2331 tracheas available for study. Epithelium 1949), and following intubation of the trachea was absent in 16 cases. This left 2170 tracheas for http://thorax.bmj.com/ (Rasche and Kuhns, 1972) and tracheostomy histological analysis. (Sara, 1967; Sara and Reye, 1969). -

Squamous Metaplasia of Normal and Carcinoma in Situ of HPV 16-Immortalized Human Endocervical Cells1
[CANCER RESEARCH 52. 4254-4260, August I, 1992] Squamous Metaplasia of Normal and Carcinoma in Situ of HPV 16-Immortalized Human Endocervical Cells1 Qi Sun, Kouichiro Tsutsumi, M. Brian Kelleher, Alan Pater, and Mary M. Pater2 Division of Basic Medical Sciences, Faculty of Medicine, Memorial University of Newfoundland, St. John's, Newfoundland, Canada A1B ÌV6 ABSTRACT genomic DNA, most frequently of HPV 16, has been detected in 90% of the cervical carcinomas and are found to be actively The importance of cervical squamous metaplasia and human papil- expressed (6, 7). HPV 16 DNA has been used to transform lomavirus 16 (HPV 16) infection for cervical carcinoma has been well human foreskin and ectocervical keratinocytes (8, 9). It immor established. Nearly 87% of the intraepithelial neoplasia of the cervix occur in the transformation zone, which is composed of squamous meta- talizes human keratinocytes efficiently, producing cell clones plastic cells with unclear origin. HPV DNA, mostly HPV 16, has been with indefinite life span in culture. Different approaches have found in 90% of cervical carcinomas, but only limited experimental data been taken to examine the behavior of these immortalized cell are available to discern the role of HPV 16 in this tissue specific onco- lines in conditions allowing squamous differentiation (10, 11). genesis. We have initiated in vivo studies of cultured endocervical cells After transplantation in vivo, the HPV 16-immortalized kerat as an experimental model system for development of cervical neoplasia. inocytes retain thépotential for squamous differentiation, Using a modified in vivo implantation system, cultured normal endocer forming abnormal epithelium without dysplastic changes at vical epithelial cells formed epithelium resembling squamous metapla early passages and with various dysplastic changes only after sia, whereas those immortalized by HPV 16 developed into lesions long periods of time in culture (10). -

Squamous Cell Carcinoma of the Breast As a Clinical Diagnostic Challenge
582 MOLECULAR AND CLINICAL ONCOLOGY 8: 582-586, 2018 Squamous cell carcinoma of the breast as a clinical diagnostic challenge KATARZYNA JAKUBOWSKA1, LUIZA KAŃCZUGA‑KODA1, WOJCIECH KISIELEWSKI2, MARIUSZ KODA3 and WALDEMAR FAMULSKI1,2 1Department of Pathomorphology, Comprehensive Cancer Center, 15‑027 Białystok; Departments of 2Medical Pathomorphology and 3General Pathomorphology, Medical University of Białystok, 15‑269 Białystok, Poland Received September 17, 2017; Accepted December 14, 2017 DOI: 10.3892/mco.2018.1581 Abstract. Squamous cell carcinoma (SqCC) of the breast metaplasia of ductal and lobular epithelial cells can be should be differentiated between the primary skin keratinizing linked with fat necrosis and infracted ademonas. Squamous squamous carcinoma and squamous metaplastic cancer. In the cell carcinoma should be differentiated between lesions of current study, the cases of two patients who were diagnosed keratinizing squamous carcinoma and squamous metaplasia with SqCC originated from skin and the breast were discussed. associated to mammary carcinoma (2). The characteristic A fine-needle aspiration biopsy confirmed the presence features of metaplastic cell carcinoma include: i) primary of atypical squamous cells. In both cases, the microscopic carcinoma without other neoplastic components such as ductal examination of the surgical specimen revealed a malignant or mesenchymal elements, ii) the tumor origin is independent neoplasm differentiated into SqCC characterized by keratin- of the overlying skin and nipple and iii) absence of primary izing cancer cells with abundant eosiphilic cytoplasm with epidermoid tumors present in other site (oral cavity, bronchus, large, hyperchromatic vesicular nuclei. Immunohistochemical esophagus, bladder, cervix ect.) (3). However, squamous studies showed negative for progesterone and estrogen recep- metaplastic carcinoma should be also differentiated with pure tors and human epidermal growth factor receptor 2. -

Taking It Step by Step: a Guide for Women Diagnosed with Gynecological Cancer
Taking it Step by Step A GUIDE FOR WOMEN DIAGNOSED WITH GYNECOLOGICAL CANCER With great thanks In This Guide Taking it Step by Step: A guide for women 1 Understanding Your Diagnosis _________ 3 diagnosed with gynecological cancer was envisioned and created by the BC/Yukon 2 Treatment Pathway & Timelines _______ 5 Women’s Cancer Information & Support Alliance. This group’s collaborative effort 3 Your Cancer Type _______________________6 includes: women with gynecological cancers; Uterine Cancer __________________________________6 the Canadian Cancer Society BC & Yukon; the Ovarian/Fallopian Tube Cancer ________________9 Gynecologic Tumour Group and Supportive Care Cervical Cancer ________________________________12 Professionals, BC Cancer Agency; Ovarian Cancer Vulvar Cancer __________________________________15 Canada Pacific Yukon Region and UBC School of Vaginal Cancer _________________________________17 Physical Therapy. The production and design for Taking it Step 4 Understanding Your Pathology Report _19 by Step was generously funded by the Cancer Program, Public Health Agency of Canada. The 5 Getting Ready _________________________20 views expressed herein do not represent the views of the Public Health Agency of Canada 6 Questions For Your Medical Team _____21 The printing and ongoing evaluation of Taking 7 Other Common Questions ____________22 it Step by Step is generously funded by the BC Cancer Foundation. The BC Cancer Foundation 8 Your Emotions __________________________23 is an independent charitable organization that raises