Comparative Fermentation Analysis of Wheat Shorts and Clear Flour to Produce Lactic Acid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pet Food & Livestock Ingredients
The Ardent Mills Advantage BROAD PORTFOLIO OF Pet Food & GRAINS, GLUTEN-FREE ORGANIC + SAFEGUARD® MICROBIAL GRAINS + PULSES NON-GMO OPTIONS REDUCTION Livestock Ingredients GROWER QUALITY + NETWORK CONSISTENCY Did You Know? QUINOA, BARLEY WHEAT FLOUR, 70% WHEAT PROTEIN & CHICKPEA FLOUR INGREDIENT are in the TOP 20 HEALTHIEST LIST CHECKING PERCEIVED IS HIGHEST AMONG INGREDIENTS PET OWNERS for consumers who look at ingredient statements 70 percent report they read the ingredient statement Source: 2019 Cargill and Ardent Mills IngredienTracker® For more information, samples, or to order, contact your Ardent Mills acount manager, visit ardentmills.com or call 800-851-9618. u Wheat-Based u Specialty & u Pulse Flours Ingredients Ancient Grains ©2019 Ardent Mills FOUNDATIONAL INGREDIENTS WHAT’S NEXT IN PET FOOD INNOVATION? FOR PET FOOD FORMULATIONS Differentiation is paramount. More than any other group of consumers, pet food owners pay attention to the ingredients in the foods they buy for their pets. Ardent Mills’ Ancient Grains and Animal lovers, farmers, trainers, and care givers want high value, quality ingredient Specialty Ingredients provide unique flavor, texture, and appearance to dry and wet pet food recipes. options to nourish their pets and livestock. Ardent Mills’ complete portfolio of Clear From biscuits, to bars, to savory treats, Ardent Mills’ extensive line of flours and grains can help deliver Flours, Middlings, Wheat Red Dog, Bran and Germ options provide a cost-effective a competitive advantage. means to deliver nutrition and value. SPECIALTY GRAINS ORGANIC Sourced from dedicated, certified organic growers, Ardent Mills Organic Flours provide the quality, consistency, and availability needed for new product launches. -

Poultry Nutrition
Poultry Science Manual, 6th edition POULTRY NUTRITION INTRODUCTION Poultry nutrition is more than just giving any available feed to your birds. Market poultry – broilers and turkeys - require proper nutrition to grow and finish out. Breeding poultry require correct nutrition to reproduce. Laying flocks require correct nutrition to be productive. As humans, we need the right balance of nutrients supplied by the food pyramid (meats, vegetables, dairy products, and fruits) on a daily basis. Similarly, poultry require the correct balance of five classes of nutrients (proteins, carbohydrates, fats and oils, vitamins, minerals, and water) for optimum growth, maintenance, finishing, work, reproduction, and production. The poultry producer must know the nutritional requirements of the bird’s function; either egg production or meat production. After determining the nutritional requirements, the poultry producer should look into the availability and cost of appropriate feedstuffs. It is also critical that the poultry nutritionist know the limitations associated with each ingredient. Some ingredients may have anti-nutritional properties which limit their usage in poultry diets. Poultry Research Feed Mill All foods that sustain life contain nutrients. Poultry convert the nutrients into useful forms via the digestive system. Blood then carries the nutrients throughout the body. Nutrients pass through capillary walls and enter body cells to provide nourishment and energy for life processes. Nutrients can be either dietary essential or non-dietary essential. Poultry feeds must supply the dietary essential nutrients because the body cannot produce them on its own. The body can synthesize the non- dietary essential nutrients for growth and maintenance. Poultry diets must supply daily nutrient requirements from the five classes of nutrients. -

Spoilage and Heating of Stored Agricultural Products
Spoilage and heating of stored agricultural products Prevention, detection, and control 1 Publication 1823E 1989 Spoilage and heating of stored agricultural products Prevention, detection, and control J.T. Millls Research Station, Winnipeg, Man. Research Branch Agriculture and Agri-Food Canada 2 ©Minister of Supply and Services Although the information contained Canada 1989 in this manual has been carefully researched, neither the author nor Available in Canada through Agriculture Canada can accept responsibility for any problems Authorized Bookstore Agents and that may arise as a result of its other bookstores application. Specific mention of a product type, brand name, or or by mail from company does not constitute endorsement by the Government of Canadian Government Publishing Canada or by Agriculture Canada. Centre Supply and Services Canada Recommendations for pesticide use Ottawa, Canada K1A 0S9 in this publication are intended as guidelines only. Any application of Price subject to change without a pesticide must be in accordance notice with directions printed on the product label of that pesticide as prescribed Cat. No. A53-1823/1988E ISBN 0 under the Pest Control Products Act. 660-13043-2 Always read the label. A pesticide should also be recommended by Staff editor provincial authorities. Because Sheilah V. Balchin recommendations for use may vary from province to province, your provincial agricultural representative should be consulted for specific advice. Canadian Cataloguing in Publication Data Mills, J.T. (John T.) Spoilage and heating of stored agricultural products: prevention, detection and control (Publication: 1823E) Includes index. Bibliography: p. 1. Farm produce -Storage - Diseases and injuries. I. Title. II. Series: Publication (Canada. -

Demand Estimation for Agricultural Processing Co-Products
Demand Estimation for Agricultural Processing Co-products Patrick Novak, Cheryl J. Wachenheim, Eric DeVuyst, and David Lambert Department of Agribusiness and Applied Economics North Dakota State University Fargo, North Dakota 58105 Presented at the Western Agricultural Economics Association Annual Meetings Logan, Utah, July 2001 Copyright 2001 by Patrick Novak, Cheryl J. Wachenheim. Eric DeVuyst, and David Lambert. All rights reserved. Readers may make verbatim copies of this document for non-commercial purposes by any means, provided that this copyright notice appears on all such copies. ABSTRACT Co-products of processing agricultural commodities are often marketed through private transaction rather than through public markets or those in which public transaction information is recorded or available. The resulting lack of historical price information prohibits the use of positive time series techniques to estimate demand. Demand estimates for co-products are of value to both livestock producers, who obtain them for use in livestock rations, and processors, who must sell or otherwise dispose of them. Linear programming has long been used, first by researchers and later as a mainstream tool for nutritionists and producers, to formulate least cost livestock rations. Here it is used as a normative technique to estimate step function demand schedules for co-products by individual livestock classes within a region. Regression is then used to smooth step function demand schedules by fitting demand data to generalized Leontief cost functions. Seemingly unrelated regression is used to estimate factor demand first adjusted for data censoring using probit analysis. Demand by individual livestock classes is aggregated over the number of livestock within a region. -

Commercial Feeding Stuffs. Report on Inspection 1934
REPORT ON INSPECTION' E. M. BMLBP Bulletin 370 March. 1935 COMMERCIAL FEEDING STUFFS REPORT ON INSPECTION 1934 E. M. BAILEY @nnttrcticut Agriritlturul Experiment Stntimt Xcm T4uocn CONNECTICUT AGRICULTURAL EXPERIMENT STATION BOARD OF CONTROL His Excellency. Governor Wilhur L. Cross, ex-oficio, Prcsiderif Elijah Rogers, Vice-Praiderrl ....................................... Southington William L. Slate, Trcasnrcr .......................................... New Haven k:dward C. Schncider, Secretory ...................................... Middletown Joseph CV. Alsop .......................................................... Avon Charles G. Morris ..................................................... Newtown Albert E. Plat~t........................................................ Braniord Olcott F. I<itl!: ...................................................South Windsor STAFF Administration. Wl!.ir~hl I.. SLICE, U.Sc., iJi?e~1~1. blass I.. 31. Iln~urrrcsr,Bookkeeper and i.ibrarian LIrrr I<nni~xn~rhl. P~~nzan,BLlrr., Ediior. ti. I<. Gnnnn~,in Chorga of B~ciidUigiand Groic,idr. Analytical i<. ki. ~I.~IL.LY,1~1t.11.. CJ~~~~~isl i% Cluvg#. Chemistry c. I<, s,,,.:~~A,<t, OWEN I.. KOLAK IIA~RVJ. PII.II. nsri.~inrlcilcmirts \I.. 'T, >,IAT,,,s IJnvro C. \vn~or~,n.S. V. I.. CIIUI<CWILL, Sampimiy1 Ancra. L'nnNr C. SIIELUON, Lobomtar)' Arriilo,zl. MRS.;\. R. \~OSBUP.ON, Secretnrs. 11. 11. \Jicn~au.P3r.D.. Bbchanxirl i$z C1zo-g~. L.~rn~*rr~11. hll~oe~, I'tr.Il., I3ercnrch Arrocintr (Yale University) GLORCER'. I'uc~~PI,PH.~).. A~~islnrlfUiocli~n~i~l. G. 1'. C~laror;,Sc.D., Bolnnirr igt Clinrge. li. &I. Siononlro. 1l.S.. Pornologist. aflra PLOXENC~A. \ICC"HMICI, PIID., P0iI8.I~ill. .\. ,\, ~>"XLAP,l',,,i>., A,,,s~,,,,, ~ll,~coloyi,,, .\. 11, AI~IIoN~ILL.l;cnert>i /f~iiil~nl. >Irs. \v. M'. I\ELS.I, Se~rcLnrg, Porcstry. \\:n~.r~a0. -

Byproducts Offer Alternatives
by Troy Smith or the producer concerned about industry, wheat midds contain higher levels “Be aware that neither wheat midd Fpossible negative effects of of fiber, protein and minerals than the pellets nor cubes will store like grain,”Blasi supplementing the cow herd’s forage-based parent grain, but have lower amounts of warns.“Producers often report problems diet with starchy feed ingredients such as starch and energy. Available as pellets or with extended on-farm storage, particularly corn or other grains, there are alternatives. cubes, the fiber-friendly nature of wheat in summer heat and humidity when the Digestible fiber energy sources, such as midds means energy may be added to pellets may soften and mold. It might be wheat middlings, corn gluten feed and forage-based diets with little or no negative best to start with small quantities until the soybean hulls, are less likely to depress impact on forage digestibility. However, producer learns how to handle the forage intake or digestibility. All of these Blasi says wheat midds generally are high in product.” feedstuffs originated as “byproducts” of phosphorous and low in calcium, so CONTINUED ON PAGE 134 grain-processing industries, but many producers may need to adjust mineral processors now refer to them as “co- supplementation accordingly. products” and have become more aggressive in their marketing efforts. According to Dale Blasi, Kansas State University (K-State) Extension specialist, the drawback to some byproduct feeds is that availability may be seasonal if processing does not occur year-round. Certain products may be most practical for producers in regions where the source crop is grown and processed, and transportation costs are low. -

Effect of Wheat Bran on Gluten Network Formation As Studied Through Dough Development, Dough Rheology and Bread Microstructure
Effect of wheat bran on gluten network formation as studied through dough development, dough rheology and bread microstructure by Hyma Gajula B.Tech., Osmania University, India, 2003 M.S., Kansas State University, 2007 AN ABSTRACT OF A DISSERTATION submitted in partial fulfillment of the requirements for the degree DOCTOR OF PHILOSOPHY Department of Grain Science and Industry College of Agriculture KANSAS STATE UNIVERSITY Manhattan, Kansas 2017 Abstract The overall hypothesis underlying this study is that the nature and extent of bran interactions with the gluten protein matrix play a dominant role in both 'in-process' dough and final product quality of whole grain baked goods. Therefore, the purposeful manipulation of those interactions should be able to minimize adverse processing or product characteristics resulting from bran inclusion/presence. The approach we took was to study the effects of bran milled to different particle sizes on dough development during and after dough mixing using fundamental rheology combined with traditional cereal chemistry approaches and x-ray microtomography (XMT). The research outcomes were used to create a better picture of how the bran is effecting the dough development and to suggest strategies that allow for the control of that effect. Study-I focused on characterization of the chemical properties, empirical rheological properties and baking performance of flours and dough with different bran contents from different sources. The development of dough microstructure and the resulting crumb texture in the presence of different bran were studied using XMT. HRW and SW bran additions resulted in higher water absorptions (WA) irrespective of the flour type and bran source. -

PDS Probreed Mix (ENG)
START AT GESTATION NOW IS THE TIME TO SWITCH Strengthens the natural resistance of the mare Highly digestible protein and energy for steady growth of the foal Mare and unborn foal need extra minerals and trace elements Mare - for her condition Foal - for strong bone development A mare needs extra nutrients from the 7th month of gestation onwards More information? Ask for it in the shop or check www.mycavalor.com Usage : Mare –7th month of gestation until lactation Foal – 1 month to 1 year old Protection of the natural immunity of the mare Pre- and probiotics for a good intestinal function Ingredients – good absorption and high digestibility Balanced composition for an even growth of the foal High protein content – development and growth of the embryo | foal for recovery of mare after pregnancy Sufficiently balanced energy for the mare Rich in minerals and trace elements Ca:P:Mg for good bone development and lactation Cu:Zn:Mn for proper development of cartilage & joints and for the mare because of protein digestion and build-up of resistance. Probreed MIX per 100 kg BW : Min. 300 g – Max. 500 g (mare) Min. 200 g – Max. 300 g (foal) Muesli available in: 20 kg XLbox 500kg art. 472349 472325 Composition: Alfalfa | wheat middlings | oats | expanded barley | expanded maize | soya feed (produced from genetically modified soya) | wheat bran | barley flakes | linseed | beet molasses | wheat gluten feed | sunflower seed feed | soya bean hulls (produced from genetically modified soya) | toasted soya beans | wheat | spelt bran | soya fibre | wheat -

Wheat and Wheat By-Products for Laying Hens W.C
South Dakota State University Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange South Dakota State University Agricultural Bulletins Experiment Station 5-1-1934 Wheat and Wheat By-Products for Laying Hens W.C. Tully J.B. Taylor Follow this and additional works at: http://openprairie.sdstate.edu/agexperimentsta_bulletins Recommended Citation Tully, W.C. and Taylor, J.B., "Wheat and Wheat By-Products for Laying Hens" (1934). Bulletins. Paper 284. http://openprairie.sdstate.edu/agexperimentsta_bulletins/284 This Bulletin is brought to you for free and open access by the South Dakota State University Agricultural Experiment Station at Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. It has been accepted for inclusion in Bulletins by an authorized administrator of Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. For more information, please contact [email protected]. Bulletin 284 May, 1934 Wheat and Wheat By-Products for Laying Hens By W. C. Tully Poultry Building, South Dakota State College Poultry Department Agricultural Experiment Station South Dakota State College of Agriculture and Mechanic Arts Brookings, South Dakota Wheat and Wheat By-Products for Laying Hens Wheat is the second largest grain crop in South Dakota and is grown extensively in many parts of the state. It has long been recognized as being second only to yellow corn as an individual grain for poultry. Wheat bran and middlings have for many years been a recognized part of starting, growing, and laying mashes for chick and poultry feeding but definite experimental work on their actual individual values seems to be lacking. -

Download Our Catalog to Learn More About Our Ingredients
ADM INGREDIENTS CATALOG FEED & PET FOOD TABLE OF CONTENTS INTRODUCTION TO ADM 1 RELIABLE, QUALITY INGREDIENT SOLUTIONS 2 WHEAT PROCESSING 3-5 OILSEED PROCESSING 10-14 Wheat Processing ______________________________ 3 Soybean Processing ___________________________ 10 Milling Products _______________________________ 4 Cottonseed Processing ______________________11-12 Wheat Middlings ______________________________ 4 Canola Processing___________________________11-12 Wheat Red Dog ________________________________ 4 Sunflower Seed Processing _____________________ 11 Corn (Maize) Hominy __________________________ 4 Flaxseed Processing ___________________________ 11 Grain Sorghum Mill Feed (Milo Hominy) ________ 4 Oilseed Products ______________________________ 13 Corn (Maize) Screenings ________________________ 4 Soybean Meal ________________________________ 13 Grains ________________________________________ 5 Soy Hulls _____________________________________ 13 Corn (Maize) Grain ____________________________ 5 Canola Meal __________________________________ 13 Milo (Grain Sorghum) __________________________ 5 Cottonseed Meal ______________________________ 13 Wheat ________________________________________ 5 Cottonseed Hulls _____________________________ 13 Barley _________________________________________ 5 Whole Cottonseed ____________________________ 13 Sunflower Meal _______________________________ 13 CORN PROCESSING 6-9 Linseed Meal _________________________________ 13 Wet Corn Milling Process _______________________ 6 Oils/Energy Products__________________________ -

Alternative Feeds for Beef Cattle FSA3047
Agriculture and Natural Resources FSA3047 Alternative Feeds for Beef Cattle Shane Gadberry Supplemental feed can be a major Supplementation Basics Professor - cost incurred to maintain beef cattle. Ruminant Nutrition Corn, grain sorghum or milo, cotton- Energy seed meal and soybean meal were traditionally used to provide supple- Total digestible nutrients make mental energy (TDN, total digestible up the majority of cattle diets. The nutrients) and protein (CP) needed by exact TDN value of a diet is difficult cattle consuming forage diets. Cattle to obtain because (1) TDN content of producers today, however, rely on an purchased feed is not displayed on abundance of alternative or byproduct feed labels, (2) TDN content derived (co-product) feeds to supplement their from a forage or feed analysis is cattle. These feeds will be described usually estimated from a prediction as alternative feeds throughout this equation, (3) TDN values for many publication. feeds change as the amount in the diet changes, especially when forage Alternative feeds may provide nutri- is replaced with alternative feeds, ents needed by cattle at a lower cost and (4) variation within the nutrient than traditional feeds. In fact, many composition of a feedstuff, as well of the locally sourced cattle supple- as how the feedstuff was stored and ments today are simple commodity processed, can affect its TDN. Cattle blends of 3 to 5 feeds. The price of producers can do little about most of these feeds is usually based on the the factors mentioned. However, cattle price of corn and a protein meal (such producers have the opportunity to as soybean meal), supply and demand have ingredients tested for nutrient and how far the feed will be trucked composition, which can be used to from its origin to its destination. -
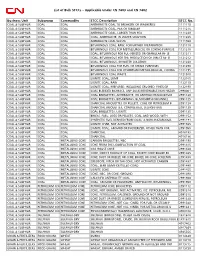
List of Bulk Stccs – Applicable Under CN 7403 and CN 7402 Business
List of Bulk STCCs – Applicable Under CN 7403 and CN 7402 Business Unit Subgroup Commodity STCC Description STCC No. COAL & SULPHUR COAL COAL ANTHRACITE COAL TO BREAKERS OR WASHERIES 1111110 COAL & SULPHUR COAL COAL ANTHRACITE COAL, PEA OR SMALLER 1111215 COAL & SULPHUR COAL COAL ANTHRACITE COAL, LARGER THAN PEA 1111220 COAL & SULPHUR COAL COAL COAL, ANTHRACITE, IN WATER SOLUTION 1111225 COAL & SULPHUR COAL COAL ANTHRACITE COAL WASTE 1111920 COAL & SULPHUR COAL COAL BITUMINOUS COAL, RAW, FORFURTHER PREPARATION 1121110 COAL & SULPHUR COAL COAL BITUMINOUS COAL FOR METALLURGICAL OR COKING PURPOSE 1121210 COAL & SULPHUR COAL COAL COAL, BITUMINOUS FOR PUL-VERIZED OR GRANULAR IN- JE 1121211 COAL & SULPHUR COAL COAL COAL, BITUMINOUS FOR THE PRODUCTION OF DIRECT RE- D 1121212 COAL & SULPHUR COAL COAL COAL, BITUMINOUS, IN WATER SOLUTION 1121220 COAL & SULPHUR COAL COAL BITUMINOUS COAL FOR FUEL OR STEAM PURPOSES 1121290 COAL & SULPHUR COAL COAL BITUMINOUS COAL FOR OTHERTHAN METALLURGICAL, COKING 1121295 COAL & SULPHUR COAL COAL BITUMINOUS COAL WASTE 1121910 COAL & SULPHUR COAL COAL LIGNITE COAL, LUMP 1122105 COAL & SULPHUR COAL COAL LIGNITE COAL, RAW 1122110 COAL & SULPHUR COAL COAL LIGNITE COAL, PREPARED, INCLUDING CRUSHED, FINES,GR 1122190 COAL & SULPHUR COAL COAL COAL BLENDED BIOMASS. ANY SOLID,RENEWABLE,NON HAZAR 2990001 COAL & SULPHUR COAL COAL COAL BRIQUETTES, ANTHRACITE, OR ANTHRACITEAND BITUM 2991120 COAL & SULPHUR COAL COAL COAL BRIQUETTES, BITUMINOUS, BLACKSMITH ORCANNEL 2991125 COAL & SULPHUR COAL COAL CHARCOAL BRIQUETTES OR PELLETS,