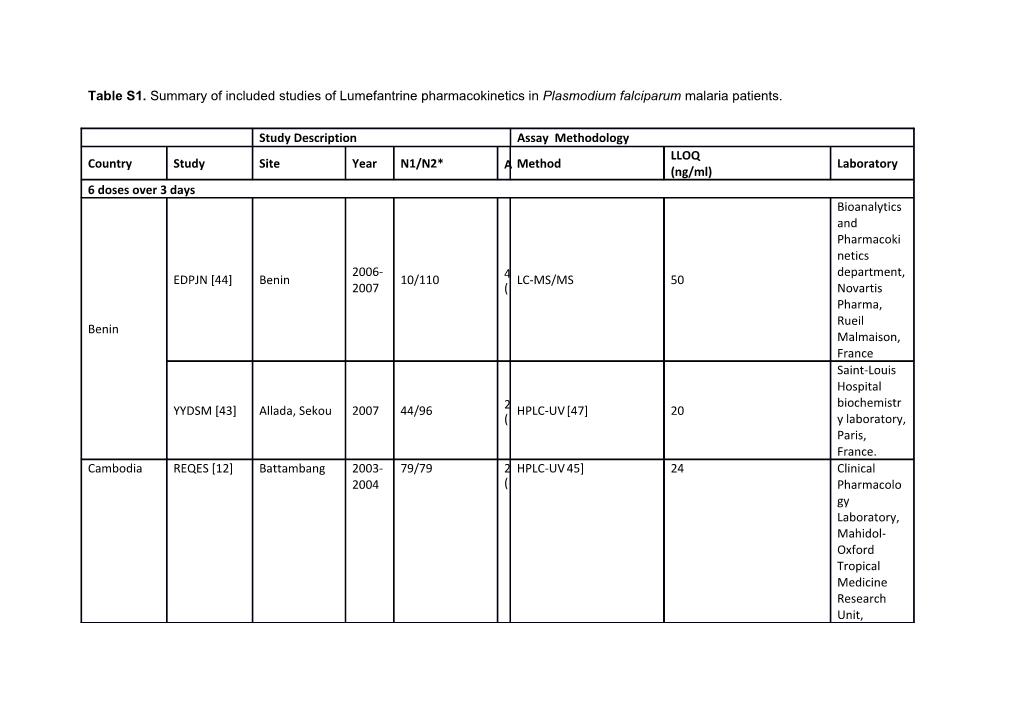
Table S1. Summary of included studies of Lumefantrine pharmacokinetics in Plasmodium falciparum malaria patients.
Study Description Assay Methodology LLOQ Country Study Site Year N1/N2* A Method Laboratory (ng/ml) 6 doses over 3 days Bioanalytics and Pharmacoki netics 2006- department, EDPJN [44] Benin 10/110 4 LC-MS/MS 50 2007 ( Novartis Pharma, Rueil Benin Malmaison, France Saint-Louis Hospital biochemistr YYDSM [43] Allada, Sekou 2007 44/96 2 HPLC-UV [47] 20 ( y laboratory, Paris, France. Cambodia REQES [12] Battambang 2003- 79/79 2 HPLC-UV 45] 24 Clinical 2004 ( Pharmacolo gy Laboratory, Mahidol- Oxford Tropical Medicine Research Unit, Thailand Bioanalytics and Pharmacoki Guinea Bandim, 2007- netic SXGQP [34] 122/191 7 HPLC-UV [47] 52.9 Bissau Belem, Cutum 2008 ( laboratory, Dalarna University, Sweden Bioanalytics and Pharmacoki netics 2006- department, EDPJN [44] Kenya 12/192 2 LC-MS/MS 50 2007 ( Novartis Pharma, Rueil Malmaison, France Kenya Clinical Pharmacolo gy Laboratory, Mahidol- QZJGM [39] Battambang 2005 101/241 3 HPLC-UV [51] 1.5 Oxford ( Tropical Medicine Research Unit, Thailand Laos HKNHR [41] Phalanxay 2002 77/110 1 HPLC-UV [51] 24 Clinical District ( Pharmacolo gy Laboratory, Mahidol- Oxford Tropical Medicine Research Unit, Thailand Service de Pharmacolo gie Clinique, 2008- FEDZY [28] Nimba County 438/502 1 HPLC-UV [47,49] 200 Hospital St. 2009 ( Vincent de Paul, Paris, France Liberia Service de Pharmacolo gie Clinique, 2008- UBTXH [38] Nimba County 106/150 3 HPLC-UV [47,49] 200 Hopital St. 2009 ( Vincent de Paul, Paris, France Bioanalytics and Pharmacoki netics department, Mali EDPJN [44] Mali 2006 8/225 3 LC-MS/MS 50 ( Novartis Pharma, Rueil Malmaison, France Mozambique EDPJN [44] Mozambique 2006 11/102 3 LC-MS/MS 50 Bioanalytics and Pharmacoki netics department, ( Novartis Pharma, Rueil Malmaison, France School of Medicine and Pharmacolo RAJDQ [35] Madang 2007 11/13 8 HPLC – UV [50] 5 gy, ( University of Western Australia, Papua New Australia Guinea School of Medicine and Pharmacolo Madang, East UANQM [33] 2005 95/128 3 HPLC-UV [48] 5 gy, Sepik ( University of Western Australia, Australia Tanzania EDPJN [44] Tanzania 2006- 19/269 3 LC-MS/MS 50 Bioanalytics 2007 ( and Pharmacoki netics department, Novartis Pharma, Rueil Malmaison, France Bioanalytics and Pharmacoki Fukayosi, 2007- netic GZQDA [32] 353/359 3 HPLC-UV [47] 25 Yombo 2008 ( laboratory, Dalarna University, Sweden Bioanalytics and Pharmacoki Fukayosi, 2007- netic KGHRT [32] 152/168 3 HPLC-UV [47] 25 Yombo 2008 ( laboratory, Dalarna University, Sweden UHUBT [42] Kilombero 2008 128 [3]/143 1 LC-MS/MS [49] 3 Division of District ( Clinical Pharmacolo gy, Department de Medicine, University Hospital and University of Lausanne, Lausanne, Switzerland Bioanalytics and Pharmacoki Fukayosi, netic XXFCZ [31] 2007 177/244 3 HPLC-UV [47] 35 Yombo ( laboratory, Dalarna University, Sweden Novartis 1997- Pharma, SMRU 66/86 2 HPLC-UV [46] 40 KGJRP [27] 1998 ( Basel, Switzerland Novartis 1996- Pharma, RGPFA [7] Bangkok 18/32 3 HPLC-UV [46] 40 1997 ( Basel, Switzerland Novartis SAUSX SMRU, Pharma, 1997 134/147 2 HPLC-UV [46] 40 [11,30] Bangkok ( Basel, Thailand Switzerland Clinical Pharmacolo gy Laboratory, Mahidol- USGDC [40] SMRU 2002 16/16 3 HPLC-UV [51] 25 Oxford ( Tropical Medicine Research Unit, Thailand Uganda CCEPC [37] Mbarara 2002- 448 [1]/957 9 HPLC-UV [48] 5 Bioanalytics 2004 ( and Pharmacoki netics department, Novartis Pharma, Rueil Malmaison, France Clinical Pharmacolo gy Laboratory, Mahidol- DBCXT [5,29] 2007- Kampala 20/579 9 HPLC-UV [51] 25 Oxford 2008 ( Tropical Medicine Research Unit, Thailand 3 doses over 3 days Clinical Pharmacolo gy Laboratory, Mahidol- Thailand USGDC [40] SMRU 2002 19/19 2 HPLC-UV [51] 25 Oxford ( Tropical Medicine Research Unit, Thailand 4 doses over 2 days Thailand FMNNB [36] SMRU 1996 43/302 2 HPLC–UV [7] 40 Novartis Pharma, ( Basel, Switzerland Novartis 1996- Pharma, KGJRP [27] Bangkok 69/85 2 HPLC–UV [46] 40 1997 ( Basel, Switzerland Novartis 1996- Pharma, RGPFA [7] Bangkok 15/33 2 HPLC-UV [46] 40 1997 ( Basel, Switzerland LLOQ: Lower Limit of Quantification; SMRU: Shoklo Malaria Research Unit, LC-MS/MS: liquid chromatography coupled with tandem mass spectrometry; HPLC-UV: High-pressure liquid chromatography with ultraviolet visible detection. N1/N2 =number of patients in PK study; N2= number of all patients in the study; number in brackets shows number of pregnant women