Key to 2nd term Examination (2008-09) S.6B ALE&T08.610a
1. PV = nRT m = RT M
mRT 0.2268.31 360 M = = PV (101103 ) (85106 ) = 78.75 (g) The molar mass of the liquid is 78.75 g
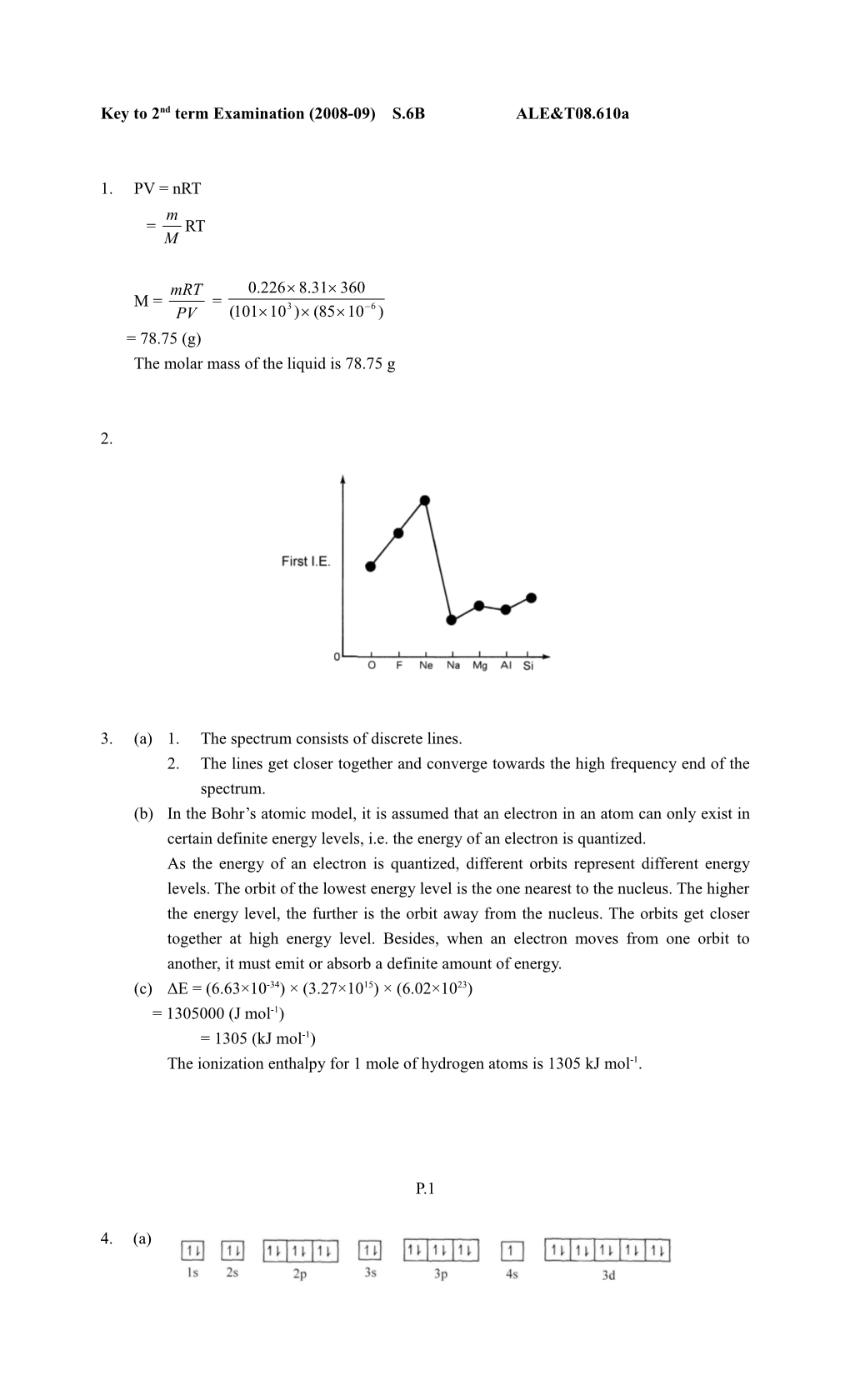
2.
3. (a) 1. The spectrum consists of discrete lines. 2. The lines get closer together and converge towards the high frequency end of the spectrum. (b) In the Bohr’s atomic model, it is assumed that an electron in an atom can only exist in certain definite energy levels, i.e. the energy of an electron is quantized. As the energy of an electron is quantized, different orbits represent different energy levels. The orbit of the lowest energy level is the one nearest to the nucleus. The higher the energy level, the further is the orbit away from the nucleus. The orbits get closer together at high energy level. Besides, when an electron moves from one orbit to another, it must emit or absorb a definite amount of energy. (c) ΔE = (6.63×10-34) × (3.27×1015) × (6.02×1023) = 1305000 (J mol-1) = 1305 (kJ mol-1) The ionization enthalpy for 1 mole of hydrogen atoms is 1305 kJ mol-1.
P.1
4. (a) (b)
5. (a) M2O(s) + CO(g) 2M(s) + CO2(g)
(b) For oxide X(M2O):
Let M1 be the molar mass of metal M. 50.8 No. of moles of M = M 1 57.2 50.8 6.4 No. of moles of O = = = 0.4 16 16 50.8 2 M = 1 1 0.4
M1 = 63.5
For oxide Y: M O Relative mass 38.1 47.7-38.1 = 9.6 38.1 9.6 Relative no. of moles 63.5 16 = 0.6 = 0.6 Mole ratio 1 1
The chemical formula of oxide Y is MO.
6. (a) A primary standard is a pure compound, it can be weighed out to make a solution of accurately known concentration. (b) A primary standard: 1. must be pure; 2. is stable in air and in solution; 3. should dissolve in water easily; and 4. should have a fairly high molar mass, so that the weighing error is reduced.
P.2
7. (a) 1. Weigh a sample of the crystals and record the reading. 2. Dissolve the crystals with some distilled water in a beaker. 3. Transfer the solution to a 250 cm3 clean volumetric flask and rinse the beaker 3 times with distilled water and also transfer the water to the flask. The solution is made up to the mark. 4. Rinse a clean pipette with the iron(II) sulphate solution. Then pipette 25.0 cm3 of this solution with pipette filler into a clean conical flask. 5. Add a few drops of 1M H2SO4 into the conical flask. 6. Rinse a clean burette with the standard potassium manganate(VII) solution and then fill up the burette with the same solution. Record the initial reading of the burette. 7. Titrate the 25.0 cm3 iron(II) sulphate solution with potassium manganate(VII) solution. End point is reached when a permanent pink colour appears. Record the final reading of the burette. 8. Repeat the titration twice. 9. Determine the percentage purity of the sample from the experimental data. Calculation: Mass of the sample = W (g) 3 Average vol. of KMnO4 used = V (dm ) -3 [KMnO4] = M (mol dm )
No. of moles of KMnO4 used = M×V 3 No. of moles of FeSO4 in 25cm solution = 5×M×V
No. of moles of FeSO4 in the sample = 50×M×V
Mass of FeSO4 in the sample = 151.8×50×M×V = 7590×MV (g) 7590MV % purity of FeSO4 in the sample = ×100% W
8. (a) No. of moles of acid/base in 50 cm3 of 2 M solution = 2 x 50/1000 = 0.1 Total volume of solution = 50 + 50 = 100 (cm3) Total mass of solution = 100 g In experiment 1:
ΔHx = 100 x 4.18 x 13.0 = 5434 J 5434 Enthalpy of neutralization ΔH1 = - 0.1 = -54340 J mol-1 = -54.34 kJ mol-1
P.3
8. (a) In experiment 2:
ΔHy = 100 x 4.18 x 10.5 = 4389 J 4389 Enthalpy of neutralization ΔH2 = - 0.1 = -43890 J mol-1 = -43.89 kJ mol-1 In the 2nd experiment, the weak acid only provides some free H+ for the reaction and the ionization of RCOOH absorbs some energy, therefore less energy is released.
+ - (b) H2O(l) H ((aq) + OH (aq) -ΔH1 = 54.6 - - RCOOH(aq)+OH (aq) RCOO (aq)+H2O(l) ΔH2 =-44.1 + - RCOOH(aq) H (aq) + RCOO (aq) ΔHd
ΔHd = 54.6 + (- 44.1) = 10.5 kJ mol-1
(c) The following assumptions have been made: (1) The heat capacity of the polystyrene cup is negligible. (2) The specific heat capacity of the solutions are similar to that of water. (3) Density of solution = Density of water = 1g cm-1 (ANY TWO)
9. (a) The entropy change of the system will be approximately zero. Reason: The total number of gaseous particles on both sides of the reactions are the same, this will not affect the disorder of the particles inside the system. (b) The entropy of the system will be negative. Reason: The total number of gaseous particles decreases, this will decrease the disorder of the particles inside the system, hence the entropy decreases. (b) The entropy of the system will be positive. 2+ 2- Reason: CuSO4(s) is a solid. In solid state, particles of CuSO4(s) (Cu and SO4 ) are 2+ 2- highly order. On the other hand, the Cu and SO4 ions are free to move in solution, hence the entropy increases.
P.4
10. (a) ΔH1: Enthalpy change of atomization (or vaporization) of calcium.
ΔH3: First ionization energy (enthalpy) of calcium.
(b) ΔH5 is the first electron affinity of oxygen. O(g) + e- O-(g) When an electron is added to the outermost shell of O(g), the attraction between the nucleus and the incoming electron outweighs the repulsion between electrons in
oxygen atom and the incoming electron, therefore, ΔH5<0.
ΔH6 is the second electron affinity of oxygen. O-(g) + e- O2-(g) After addition of an electron, the size of the oxygen anion expands, the nuclear attraction for another incoming electron is weakened. Moreover, it has become very difficult to force a negative electron onto an already negative ion, owing to
electrostatic repulsion, therefore, ΔH5>0
As a result, ΔH5 and ΔH6 have different signs. (c) (By Hess' Law)
+177 + 250 +590+1100+ (-141) + 791 +ΔHlattice[CaO(s)] =-635
ΔHlattice[CaO(s)] = -635-177-250-590-1100+141-791 = -3402 (kJ mol-1) (d) The first statement “MgO (s) has more negative lattice enthalpy.” is true. The size of the Mg2+ is smaller than that of Ca2+, there is stronger electrostatic attraction between cations and anions. Therefore, MgO(s) has more negative lattice enthalpy. On the other hand, the thermal stability of a compound depends on its enthalpy change of formation rather than on its lattice enthalpy. In this case, MgO(s) has more negative lattice enthalpy, the enthalpy change of formation is dominated by this lattice enthalpy, therefore, it is likely true that MgO(s) is more energetically stable than CaO(s).
11. (a) ΔG = ΔH - TΔS 165 ΔG = +178 – 298× = +128.8 kJ mol-1 1000 (b) ∵ΔG>0
∴CaCO3(s) does not decompose spontaneously at 298K.
(c) CaCO3(s) will decompose spontaneously if ΔG<0. i.e. ΔH – TΔS < 0 178 – T × 0.165 < 0 178 < 0.165T 178 T > 0.165 T > 1079 (K)
∴ The minimum temperature for causing the thermal decomposition of CaCO3(s) would be 1079K. P.5
o 12. (a) CH4(g) C(graphite) + 2H2(g) ΔH =+75.0 o C(graphite) + O2(g) CO2(g) ΔH =-393.5 o + 2H2(g) + O2(g) 2H2O(l) ΔH =-285.9x2 ------o CH4(g) + 2O2(g) 2H2O(l) + CO2(g) ΔH298 o ΔH298 = 75 + (-393.5) + (-285.9x2) -1 = -890.3 (kJ mol ) (b) Vaporization of water: o H2O(l) H2O(g) ΔH vap From (a)
2H2O(l) + CO2(g) CH4(g) + 2O2(g)---(1) 890.3 From (b)
CH4(g) + 2O2(g) 2H2O(g) + CO2(g)---(2) -801.7 o (1) + (2): 2H2O(l) 2H2O(g) ΔH1 o ΔH1 = 890.3 + (-801.7) = 88.6 o ΔH vap = 88.6/2 -1 = 44.3 (kJ mol )
(c) C(graphite) + O2(g) CO2 ---(1) -393.5
C(diamond) + O2(g) CO2(g) ---(2) -395.4
CO2(g) C(diamond) + O2(g) ---(3) +395.4 o (1)+(3): C(graphite) C(diamond) ΔH2 o -1 ΔH2 = -393.5 + 395.4 = +1.9 (kJ mol ) The energy required for conversion of graphite to diamond is +1.9 kJ mol-1. So graphite is energetically more stable than diamond. At room temperature, diamond is kinetically stable with respect to graphite, because the activation energy for the conversion of diamond to graphite is very large so that diamond does not convert to graphite.
13. Sum of bond enthalpies of reactants = 3×(+348) + 10×(+412) = +5164 (kJ mol-1) ∴Energy absorbed = +5164 kJ mol-1 Sum of bond enthalpies of products = (+612) + (+348) + 10×(+412) = +5080 (kJ mol-1) ∴Energy released = +5080 kJ mol-1
ΔHR = (5164)+(-5080) = +84 (kJ mol-1)
P.6
14. (a) (i) A. (ii) A has a 3-dimensional network structure, all the bonds in A are equally strong C- C covalent bonds. The structure cannot be broken without breaking these strong bonds. (or a large amount of energy is required to break these bonds) (b) (i) E. (ii) Although the bonds within each E molecules are strong covalent bonds, the forces between the molecules are only weak instantaneous dipole-induced dipole attractions (van der Waals' forces). At room temperature E turns readily into a gas. (c) (i) C. (ii) Strong force of the hammer causes movement of the planes of ions within the crystal. This results in significant repulsion between planes of ions with the same charges, causing them to fly apart. (d) (i) B. (ii) Ions in B are surrounded by a delocalized sea of electrons, which can move in the direction of an externally applied potential difference.
15. The descending order of boiling points is:
LiCl > CCl4 > BCl3 LiCl has the highest boiling point among the three substances. The reason is that the Li+ and Cl- ions are held together in the giant lattice by strong ionic bonds. A relatively large amount of energy is required to overcome the ionic bonds in the process of boiling. On the other
hand, as both CCl4 and BCl3 are simple molecular substances, the molecules are held together by instantaneous dipole-induced dipole attractions (van der Waals' forces) only. Therefore, a relatively small amount of energy is needed to overcome these attractions, and hence they have a lower boiling point than LiCl.
The reason why CCl4 has a higher boiling point than BC13 is that CCl4 has a larger
molecular size than BCl3. So the instantaneous dipole-induced dipole attractions between the CCl4 molecules are stronger than those between the BCl3 molecules.
16. (a) V-shaped
S N N F S F It possesses a dipole moment.
(b) See-saw shaped
O Xe O
F F It possesses a dipole moment.
P.7
1 1 17. (a) No. of C60 in a unit cell = 8× + 6× = 4 8 2 No. of C atoms in a unit cell = 4×60 = 240 (b) 12 (c) 1. They are soluble in organic solvent. 2. They are extremely hard. 3. They can conduct electricity. 4. They can house various metal atoms inside, or have different metals attached to the outside. 5. They can act as a catalyst. 6. They can undergo addition reaction. (Any THREE) (d) 1. lubricant. 2. superconductor. 3. rocket fuel. 4. laser material. 5. magnetic film. 6. cancer and AIDS drugs. 7. catalysts. 8. adsorbents 9. photoelectric material. (Any THREE)
18. (a) The relative molecular mass of benzoic acid (C6H5COOH) is 122. In hexane, benzoic acid molecules form dimmers with hydrogen bonds between the molecules. Therefore, benzoic acid has an apparent relative molecular mass of 244.
O...... H O C C O H ...... O However, in water, the benzoic acid molecules form hydrogen bonds with the water molecules, benzoic acid exists as monomer in aqueous solution, hence its relative molecular mass is 122.
H O...... H O. .. C H O H ...... O H
(b) Cyclohexane is non-polar, its molecules do not form hydrogen bonds with water. On the other hand, glucose can form hydrogen bonds with water molecules via it –OH groups. Therefore, glucose is soluble in water but cyclohexane is not. P.8
19. (a) TA: Sublimation curve.
Along this curve, solid and vapour CO2 coexist and in equilibrium.
(b) C is the critical point. At critical point, the density of liquid CO2 and gaseous CO2 are equal, the liquid and gaseous phases become identical and indistinguishable, the phase boundary disappears. Above the critical point, the average kinetic energy of the molecules is so high that the vapour cannot be condensed no matter what pressure is applied. (c) Along TB, there is an equilibrium.
CO2(s) CO2(l)
As CO2(s) is denser than CO2 (l), for the same amount of CO2, the volume of CO2(l)
is larger than the volume of CO2(s), by Le Chatelier’s principle, an increase in
pressure would shift the equilibrium to the left, more CO2(s) will be formed at
equilibrium. Therefore, the melting point of CO2(s) increases with an increase in external pressure, hence, BD has a positive slope. This is a typical behaviour of most material.
20. (a) 3Cu(s) + 8HNO3(aq) 3Cu(NO3)2(aq) + 4H2O(l) + 2NO(g)
(b) Cl2O6(l) + H2O(l) HClO3(aq) + HClO4(aq)
21. (a) As compared with Na+, Mg 2+ has a higher charge density. This high charge density of Mg2+ causes a great distortion of the electron cloud in the Br- ion. i.e. Mg2+ polarizes Br- to a greater extent. The ionic bonds formed by Mg 2+ and Br- have a larger covalent
character. Therefore the boiling point of MgBr2 is lower that that of NaBr. heat (b) MgCO3 MgO + CO2 As compared with Ba2+, Mg 2+ has a smaller size and higher charge. i.e. higher charge 2- density. The CO3 is polarized to a greater extent in MgCO3. Hence, it decomposes with greater ease and therefore at lower temperature.
22. (a) (i) Dipole moment (μ) is a measure of the polarity of a molecule. It is expressed as a product of charge(q) on an atom and internuclear distance(d), it reflects both the quantity of charge involved and the distance of separation. i.e. μ = q × d (ii) Bond dissociation enthalpy of a diatomic molecule H-X is the enthalpy change (or energy required) when 1 mole of H-X bond is broken. (b) The electronegativity of F is much greater than that of I. In the case of HF and HI, though the distance between H and I is greater than that of H and F, the effect of electronegativity difference outweighs the effect of difference in internuclear distance. Therefore, HF has a greater dipole moment than HI. The atomic radius of F is much smaller than that of I, attraction of atomic nuclei on bonding electrons is much stronger in the case of HF, therefore, it takes more energy to break the H-F bond and HF has a greater bond dissociation enthalpy. P.9
23. (a)
(b)
24. The solubility of the Group II sulphates decreases down the group. BeSO4 is very soluble in
water while BaSO4 is insoluble. Solubility can be related to: 1. hydration energy: Going down the group, the increasing size of the cation lowers the hydration energy. The Be2+ ion, being the smallest, produces the most hydration energy, it is thus the most soluble. Ba2+, being the largest ion, is the least soluble. 2. lattice energy: The sulphate of smaller cations are more stable, they have higher lattice energy. The larger the lattice energy, the more strongly the ions are bonded, and this decreases solubility. i.e. Solubility increases down the group if we consider lattice energy only. In practice, for the sulphates of group II metals, the cations are much smaller than the anions. Down the group, the change in size of the cations does not cause a significant change in lattice energy. i.e. solubility depends mostly on hydration energy. Mathematically,
ΔHsolution = ΔHhydration – ΔHlattice P.10
1 24. ΔHlattice where r+ and r- are the radius of cation and anion respectively. r r 2- For SO4 (large ion), r- >>r+, r- + r+ ≈ r- 1 ∴ ΔHlattice r 1 i.e. ΔHlattice = k = constant r
ΔHsolution = ΔHhydration – constant Therefore the solubility of group II sulphate is affected more by the hydration energy of its cations than its lattice energy, as a result, the solubility of the Group II sulphates decreases down the group.
25. (a) (i) A disproportionation is a redox reaction in which the same species under oxidation and reduction simultaneously.
(ii) Cl2(g) + 2NaOH (cold and dilute) NaOCl(aq) + NaCl(aq) + H2O(l) (b) 0.1 M HF(aq) is weaker acidic than 0.1M HCl(aq) because of the very high H-F bond + - energy, this makes the ionization of HF into H3O and F less favourable. The currently accepted theory is that the H+ are involved in hydrogen bonding to the HF to form tight ion pair. A HF is such a far better participant in hydrogen bonding + + than a HCl. The concentration of H (H3O ) is lowered, hence, the acidity is weaker.
+ H F ...... H
HF H+ +F- + + HF+ H H2F + - Overall: 2HF H2F + F
26. (a) (i) SiO2 has a giant covalent lattice which needs to take in a lot of energy on melting.
However SO3 is a discrete molecule and the interaction among molecules is weak
van der Waals' force. As a result, low melting point is expected for SO3. (ii) The charge on Mg2+ is +2 whereas on Na+ is +1. The ionic radius of Mg2+ is + shorter than Na , hence, MgO has a higher lattice energy than Na2O, higher lattice energy results in higher melting point. (b) According to the melting point listed in the given table.
Al2O3 is an ionic compound with covalent character, and P4O10 is a typical covalent molecule. (c) Melting point reflects the bonding and structure of compounds (consists of molecules or ions or atoms; three dimensional network or discrete molecules; face centred cubic or body centred cubic; covalent bond v.s. ionic bond and etc.). Based on the melting point only, it is impossible to compare the strength of Mg-O bond in MgO and Si-O
bond in SiO2. P.11
27. By adding an acidified silver nitrate(V) to the solution of the tested samples, then observing the colour of precipitates of silver halides. Silver chloride is a white precipitate. Silver bromide is a pale yellow precipitate and silver iodide is a yellow precipitate. The nature of silver halides can be confirmed by adding excess ammonia solution into the precipitates of silver halides, if the tested silver halide is a silver chloride, it dissolves in ammonia solution rapidly. Silver bromide dissolves in ammonia solution slowly and silver iodide is insoluble in ammonia solution.
P.12