Clinical Communications Symmetrical Drug
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Symmetrical Intertriginous and Flexural Exanthema Due to Bortezomib (A Proteasome Inhibitor) Given for Myeloma
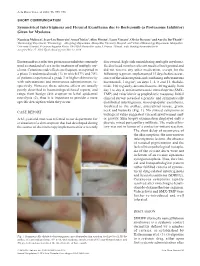
Acta Derm Venereol 2016; 96: 995–996 SHORT COMMUNICATION Symmetrical Intertriginous and Flexural Exanthema due to Bortezomib (a Proteasome Inhibitor) Given for Myeloma Nausicaa Malissen1, Jean-Luc Bourrain1, Anca Chiriac2, Aline Montet1, Laure Vincent3, Olivier Dereure1 and Aurélie Du-Thanh1* 1Dermatology Department, 2Pneumology – Allergology Department, Montpellier University Hospital, and 3Clinical Hematology Department, Montpellier University Hospital, 80 avenue Augustin Fliche, FR-34295 Montpellier cedex 5, France. *E-mail: [email protected] Accepted Mar 17, 2016; Epub ahead of print Mar 22, 2016 Bortezomib is a selective proteasome inhibitor currently discovered, high-risk smouldering multiple myeloma. used as standard of care in the treatment of multiple my- He disclosed no other relevant medical background and eloma. Cutaneous side-effects are frequent, as reported in did not receive any other medication, except for the a phase 3 randomized study (1), in which 57% and 70% following regimen, implemented 15 days before occur- of patients experienced a grade 3 or higher skin toxicity rence of the skin eruption and combining subcutaneous with subcutaneous and intravenous administration, re- bortezomib, 1 mg/m2, on days 1, 4, 8 and 11, thalido- spectively. However, these adverse effects are usually mide, 100 mg daily, dexamethasone, 40 mg daily from poorly described in haematological-based reports, and day 1 to day 4, sulfamethoxazole trimethoprim (SMX- range from benign skin eruption to lethal epidermal TMP) and valaciclovir as prophylactic measures. Initial necrolysis (2), thus it is important to provide a more clinical survey revealed a pruritic and symmetrically specific description when they occur. distributed intertriginous, maculopapular exanthema, localized to the axillae, antecubital fossae, groin, neck and buttocks (Fig. -

Symmetrical Drug-Related Intertriginous and Flexural Exanthema Secondary to Topical 5-Fluorouracil
Symmetrical Drug-Related Intertriginous and Flexural Exanthema Secondary to Topical 5-Fluorouracil Roxann Powers, MD; Rachel Gordon, MD; Kenrick Roberts, MD; Rodney Kovach, MD We report the case of a 56-year-old man who fossae and axillae. His dermatologist stopped the developed a distinctive skin eruption after treat- 5-FU and started prednisone, erythromycin, hydro- ing actinic keratoses on the dorsal aspects of cortisone ointment, and fexofenadine hydrochloride. his right and left hands with topical 5-fluorouracil Over the next week, the areas became eroded and the (5-FU). The distribution of his rash was charac- pain worsened. A biopsy was performed. The hydro- teristic of symmetrical drug-related intertriginous cortisone was discontinued and he was referred for a and flexural exanthema (SDRIFE), also known as second opinion. baboon syndrome. Medications at presentation included erythromycin, Cutis. 2012;89:225-228. fexofenadine hydrochloride, acetaminophen-codeine, CUTISprednisone, petrolatum ointment, metformin Case Report hydrochloride, enalapril maleate, ranitidine hydro- A 56-year-old man with a history of diabetes mel- chloride, simvastatin, triamterene/hydrochlorothiazide, litus, hypertension, hyperlipidemia, reflux, squamous aspirin, and omeprazole. He reported no cell carcinoma in situ of his right hand, and actinic known allergies. keratoses of the right Doand left hands presentedNot with a PhysicalCopy examination revealed an overweight painful, burning, pruritic, erythematous, and blister- man who appeared agitated and reported substantial ing rash on his medial thighs, scrotum, penis, antecu- discomfort. He had well-demarcated, violaceous, red bital fossae, axillae, and dorsal hands. The patient had erosions on his medial thighs (Figure 1), scrotum, a successful history of treatment of actinic keratosis and penis; erythematous denuded patches in the on his right hand with topical 5-fluorouracil (5-FU) antecubital fossae (Figure 2) and axillae; and crusty 8 years prior to presentation. -

Not Just Luck
EDITORIAL Not Just Luck few months ago, I described a 52-year-old and encourage potential authors and reviewers. At a female patient’s puzzling rash to a colleague: memorable meeting last winter, during a howling snow- a striking symmetrical, bright-red, sharply- storm one dark Thursday night, I met Susan Busch and defined macular erythema of the genital/ Jane Tallent, the 2009Y2010 Lahey Clinic Dermatology inguinal and medial-gluteal areas, and much Nurse Practitioner Fellowship fellows, quintessential ex- less prominently, the axillae and inframmary folds. Sitting amples of individuals who heartily seek out learning op- Aon her desk was an article, just read, describing this un- portunities. We talked animatedly about ways they might usual drug eruption. Coined the Bbaboon syndrome[ be- contribute to the Journal, and I forgot how sweet it would cause of the resemblance to the red buttocks of baboons, it have been to stay home and light a fire in the fireplace. has been renamed the less colorful, less memorable, but less- When an uncanny opportunity presents itself to apply some- unnerving-to-be-diagnosed- thing we have just learned, we can remind ourselves that we with Bsymmetrical drug-related helped create this fortunate situation; we helped create our intertriginous and flexural exan- own luck. A dermatology nurse approached me at the 2009 thema[ (SDRIFE; Hausermann, Dermatology Nurses’ Association summer meeting in Boston, Harr, & Bircher, 2004). reporting that only a day or two after she was introduced to I said it was good luck that Merkel cell carcinoma by the JDNA article by Victoria she had just read this article, and Garcia-Albea (nee Beebe; Beebe, 2009), she heard colleagues a colleague commented, para- discussing a new patient diagnosed with Merkel cell cancer in phrasing Louis Pasteur, BChance their practice. -

Skin Allergy to Azole Antifungal Agents for Systemic Use: a Review of the Literature
Send Orders for Reprints to [email protected] Recent Patents on Inflammation & Allergy Drug Discovery 2019, 13, 1-14 1 REVIEW ARTICLE Skin Allergy to Azole Antifungal Agents for Systemic Use: A Review of the Literature Gianfranco Calogiuri1,3, Lene H. Garvey2, Eustachio Nettis3, Paolo Romita4, Elisabetta Di Leo5, Riccardo Caruso6, Lavjay Butani7 and Caterina Foti4,* 1Department of Pneumology and Allergy Hospital Sacro Cuore - Gallipoli, Lecce, Italy; 2Allergy Clinic, Department of Dermato-Allergology, Herlev and Gentofte Hospital, University of Copenhagen, Hellerup, Denmark; 3Department of Biomedical Sciences and Human Oncology Unit of Internal Medicine "G. Baccelli", "Aldo Moro" University of Bari Medical School, Policlinico, Bari, Italy; 4Department of Biomedical Science and Human Oncology, Dermatological Clinic, University of Bari, Bari, Italy; 5Section of Allergy and Clinical Immunology, Unit of Internal Medicine-"F. Miulli" Hospital, Acquaviva delle Fonti, Bari, Italy; 6Clinica Salus - Brindisi, Brindisi, Italy; 7Section of Pediatric Nephrology, Department of Pediatrics, University of California Davis Medical Center, Sacramento, CA, USA Abstract: Background: Antifungal azoles are the first-line agents used to treat topical and, above all, systemic mycosis. The latter could be life-threating infections in immunocompromised patients. Che- motherapeutic antibiotics, including antifungal azoles, may induce hypersensitivity reactions; however, such immunologic adverse reactions have not been defined and carefully investigated. Objective: The study aimed to provide an update on the evaluation and diagnosis of skin allergy to azole antifungal agents. Methods: This is a systematic review performed on PubMed and Google Schoolbarusing using the key terms “allergy, hypersensitivity, anaphylaxis, immediate-type reaction, delayed-type reaction, keto- conazole, fluconazole, posaconazole, voriconazole, itraconazole, triazoles, imidazoles, antifungals, A R T I C L E H I S T O R Y antimycotics”. -

General Aspects 14 Niels K
14_199_254* 05.11.2005 10:15 Uhr Seite 201 Chapter 14 General Aspects 14 Niels K. Veien Contents 14.1 Introduction . 201 14.4.2.1 Cement Ulcerations . 224 14.4.2.2 Pigmented Contact Dermatitis . 225 14.2 The Medical History of the Patient . 202 14.4.2.3 Caterpillar Dermatitis and Irritant Dermatitis 14.2.1 History of Hereditary Diseases . 202 from Plants and Animals . 225 14.2.2 General Medical History . 202 14.4.2.4 Head and Neck Dermatitis . 225 14.2.3 History of Previous Dermatitis . 203 14.4.2.5 Dermatitis from Transcutaneous Delivery 14.2.4 Time of Onset . 203 Systems . 225 14.2.5 History of Aggravating Factors . 204 14.4.2.6 Berloque Dermatitis . 226 14.2.6 Course of the Dermatitis . 205 14.4.2.7 Stomatitis due to Mercury or Gold Allergy . 226 14.2.7 Types of Symptoms . 205 14.5 Regional Contact Dermatitis . 226 14.3 Clinical Features of Eczematous Reactions . 206 14.5.1 Dermatitis of the Scalp . 226 14.3.1 Acute and Recurrent Dermatitis . 206 14.5.2 Dermatitis of the Face and Neck . 228 14.3.2 Chronic Dermatitis . 210 14.5.2.1 The Lips . 230 14.3.3 Nummular (Discoid) Eczema . 211 14.5.2.2 The Eyes and Eyelids . 230 14.3.4 Secondarily Infected Dermatitis . 211 14.5.2.3 The Ear . 231 14.3.5 Clinical Features of Contact Dermatitis 14.5.3 Dermatitis of the Trunk . 232 in Specific Groups of Persons . 212 14.5.3.1 The Axillary Region . -

A New Proposal for a Clinical-Oriented Subclassifi- Cation of Baboon Syndrome and a Review of Baboon Syndrome
Original article A new proposal for a clinical-oriented subclassifi- cation of baboon syndrome and a review of baboon syndrome Atsushi Miyahara1, 2, Hisashi Kawashima2, Yukari Okubo3 and Akinori Hoshika2 drug-related intertriginous and flexural exanthema Summary (SDRIFE), classical baboon syndrome, topical Objective: To review baboon syndrome (BS). drug-induced baboon syndrome, systemic drug- Data Sources: Date sources were obtained from induced baboon syndrome, recall phenomenon, p-i PubMed and Google Scholar: Photographs of (pharmacologic interaction with immuno-receptors) baboon syndrome were obtained from our concept. patient. Study Selections: PubMed and Google Scholar Introduction were searched up to June 30, 2010. The search The term “baboon syndrome”(BS) was terms were “baboon syndrome”, “SDRIFE” and introduced in 1984 to describe a mercury-induced “thimerosal allergy”. Reverse references from characteristic eruption with previous sensitization to relevant articles and Google Scholar were also mercury1. It presents with a diffuse symmetrical used. As BS is a classical disease and cases of erythema, predominantly on major flexural areas, offending agents were relatively old, some and an inverted triangular or V-shaped erythema on references were more than five years old. In both upper antero-medial thighs1. BS was named order to gather as many cases of offending agents after the red bottomed baboon1. as possible, more than 50 references were Recently it has been revealed that an eruption collected. which resembles mercury-induced BS was due not Results and Conclusion: We divided BS into as 4 only to mercury but also other metals, drugs and groups; classical baboon syndrome, topical drug- natural products, with or without previous induced baboon syndrome, systemic drug- sensitization to offending or related drug(s). -

Presenters: Philip R
UC Davis Dermatology Online Journal Title Zoledronic acid-associated symmetrical drug-related intertriginous and flexural exanthema (SDRIFE): report of baboon syndrome in a woman with recurrent metastatic breast cancer after receiving zoledronic acid Permalink https://escholarship.org/uc/item/5kk0g864 Journal Dermatology Online Journal, 21(8) Author Cohen, Philip R Publication Date 2015 DOI 10.5070/D3218028425 License https://creativecommons.org/licenses/by-nc-nd/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Volume 21 Number 8 August 2015 Case report Zoledronic acid-associated symmetrical drug-related intertriginous and flexural exanthema (SDRIFE): report of baboon syndrome in a woman with recurrent metastatic breast cancer after receiving zoledronic acid Philip R Cohen MD Dermatology Online Journal 21 (8): 2 Department of Dermatology, University of California San Diego, San Diego, California. Correspondence: Philip R. Cohen, MD 10991 Twinleaf Court San Diego, CA 92131-3643 [email protected] Abstract Background: Baboon syndrome is a distinctive skin reaction in which the patient typically develops erythematous buttocks that appear similar to those of a baboon. The non-contact allergenic variant of baboon syndrome is also referred to as symmetrical drug-related intertriginous and flexural exanthema (SDRIFE). Zoledronic acid is a bisphosphonate that is used in patients with metastatic cancer to prevent bone complications. Purpose: Zoledronic acid-associated baboon syndrome is described in a woman with recurrent metastatic breast cancer. Methods: PubMed was used to search the following terms, separately and in combination: baboon syndrome, breast cancer, symmetrical drug-related intertriginous and flexural exanthema, and zoledronic acid. -

Systemic Drug Related Intertriginous and Flexural
Available online at www.sciencedirect.com www.jdds.org ScienceDirect Journal of Dermatology & Dermatologic Surgery xxx (2016) xxx–xxx Systemic drug related intertriginous and flexural exanthem (SDRIFE) due to clindamycin Dear Editor, Discussion A 50-year-old male was referred to us for a symmetric, confluent, erythematous, pruritic eruption of a three day Systemic drug-related intertriginous and flexural exan- duration involving the flexures, which appeared two days thema (SDRIFE) is a distinctive drug eruption typically after starting intravenous clindamycin for chronic involving the flexural folds and gluteal areas, commonly osteomyelitis. There was no past history of any similar observed after exposure to beta-lactam antibiotics, such rash. Cutaneous examination revealed the presence of well as penicillins and cephalosporins, with amoxicillin being defined maculopapular eruption involving the axillae, cubi- implicated in the majority of cases (Ha¨usermann et al., tal fossa, inguinal regions and buttocks (Fig. 1a, b). There 2004). was no associated fever or lymphadenopathy. The mucosae SDRIFE is thought to result from a type IV delayed and other system examination were normal. Blood counts, hypersensitivity immune response to the offending drug. liver and renal function tests were normal as were the chest Occlusion, sweating or frictional injury and recall X-ray and abdominal ultrasound. There was no atypical phenomenon of past dermatitis have also been presumed lymphocytosis or eosinophilia. A biopsy was advised but to play a role in its pathogenesis (Kardaun and Tupker, was refused by the patient. 2012). Few authors have also postulated recall phe- Causality assessment was carried out using the Naran- nomenon as a cause of SDRIFE. -

Malignant Intertrigo: a Subset of Toxic Erythema of Chemotherapy Requiring Recognition
CASE SERIES Malignant intertrigo: A subset of toxic erythema of chemotherapy requiring recognition SabrinaM.Smith,MD,a Philip B. Milam, MD,a Stephanie K. Fabbro, MD,a AlejandroA.Gru,MD,b and Benjamin H. Kaffenberger, MDa Columbus, Ohio and Charlottesville, Virginia Key words: chemotherapy; drug eruption; graft-versus-host disease; intertrigo; pathogenesis; toxic erythema of chemotherapy. INTRODUCTION Abbreviations used: Toxic erythema of chemotherapy (TEC) encom- passes a broad spectrum of cytotoxic effects on the MI: malignant intertrigo 1 PPE: palmoplantar erythrodysesthesia skin. Despite increasing awareness of TEC, such SDRIFE: symmetrical drug-related intertriginous, eruptions may remain unrecognized and subse- flexural exanthema quently result in unnecessary hospitalizations. Of TEC: toxic erythema of chemotherapy the different variants of TEC, intertriginous eruptions have been reported using many terms, such as intertrigo dermatitis, intertriginous eruption of REPORT OF CASES chemotherapy, flexural erythematous eruption, and Six patients with various malignancies undergo- intertrigo-like eruption associated with chemo- ing chemotherapy presented with intertriginous therapy.1 Intertrigo is a broad term that refers to rashes (Table I and Fig 1). Involved areas included any inflammatory rash of closely opposed skin, cervical, inframammary and inguinal folds, and typically presenting with varying degrees of ery- axillae, abdomen, antecubital fossae, perineum, thema and maceration, and this particular type of buttocks, and thighs. Physical examination found TEC has not been identified with consistent nomen- sharply demarcated, erythematous-to-dusky patches clature. We present 6 cases of this distinct subset of and plaques with focal scaling, crusting, and ero- TEC, all occurring within a 7-month period at a single sions. These lesions were painful and exquisitely institution; these cases were initially unrecognized, tender. -

Symmetric Drug-Related Intertriginous and Flexural Exanthema
CASE LETTER Symmetric Drug-Related Intertriginous and Flexural Exanthema Dylan Haynes, MD; Zachary Pena, MD; Kevin White, MD; Tracy Funk, MD; Jesse J. Keller, MD A 39-year-old woman who was otherwise healthy PRACTICE POINTS presented to the emergency department after developing a rapidly evolving andcopy blistering rash on the left flank. • Symmetric drug-related intertriginous and flexural exanthema (SDRIFE) appears in the Hours later, the rash had progressed to a sharply demar- absence of systemic signs and symptoms such cated, confluent, erythematous plaque with central ulcer- as fever, which may help differentiate it from infec- ation and large flaccid bullae peripherally, encompassing tious causes. 18% of the total body surface area and extending from the gluteal cleft to the tip of the scapula along the left • β-Lactam antibiotics, particularly amoxicillin, not are common offenders in the pathogenesis of flank (Figure 1) with no vaginal or mucosal involvement. SDRIFE, but new drug relationships frequently are The patient recently had completed a 10-day course of being described. amoxicillin–clavulanic acid 2 days prior for a cat bite on • Symmetric drug-related intertriginous and the right dorsal wrist. Additional history confirmed the flexural exanthema commonly follows a benign Doabsence of prodromal fever, fatigue, or chills. Inciting course but warrants respect, as it may have devas- trauma including chemical and thermal burns was denied. tating potential. Potential underlying psychosocial confounders were explored and were unrevealing. Laboratory test results including a complete blood cell count and metabolic panel as well as vital signs were unre- To the Editor: markable except for slight leukocytosis at 14,000/µL (refer- Symmetric drug-related intertriginous and flexural exan- ence range 4500–11,000/µL). -

The Use of Glycopyrrolate in Aquagenic Wrinkling of the Palms Helia Eragi, D.O., Mayha Patel, B.S., MSIV, David Horowitz, D.O., F.A.O.C.D
V Journal of the American Osteopathic College of Dermatology PRSRT STD OLUME U.S. POSTAGE Volume 25 2902 North Baltimore Street PAID 25 MASON CITY, IA P.O. Box 7525 PERMIT NO. 429 Kirksville, Missouri 63501 Journal of the American Osteopathic College of Dermatology J OURNAL OF THE A MERICAN O STEOPATHIC C OLLEGE OF D ERMATOLOGY What Dermatologists Need to Know About Interferon- gamma Testing for Tuberculosis 1 ournal of the American Osteopathic J College of Dermatology 2012-2013 OFFICERS PRESIDENT David L. Grice, D.O., FAOCD PRESIDENT-ELECT Suzanne Sirota-Rozenberg, D.O., FAOCD FIRST VICE-PRESIDENT Rick J. Lin, D.O., FAOCD SECOND VICE-PRESIDENT Alpesh Desai, D.O., FAOCD THIRD VICE-PRESIDENT Karthik Krishnamurthy, D.O., FAOCD IMMEDIATE PAST-PRESIDENT Editor-in-Chief Bradley Glick, D.O., FAOCD Karthik Krishnamurthy, DO TRUSTEES Danica Alexander, D.O., FAOCD Reagan Anderson, D.O., FAOCD Mark A. Kuriata, D.O., FAOCD Sponsors: Daniel Ladd, D.O., FAOCD Bayer John P. Minni, D.O., FAOCD Bryan Sands, D.O., FAOCD Global Pathology Laboratory SECRETARY-TREASURER Medicis Jere J. Mammino, D.O., FAOCD EXECUTIVE DIRECTOR Ranbaxy Marsha A. Wise, B.S. JAOCD Founding Sponsor AOCD • 2902 N. Baltimore St. • Kirksville, MO 63501 800-449-2623 • FAX: 660-627-2623 www.aocd.org COPYRIGHT AND PERMISSION: Written permission must be obtained from the Journal of the Ameri- can Osteopathic College of Dermatology for copying or reprinting text of more than half a page, tables or figures. Permissions are normally granted contingent upon similar permission from the author(s), inclusion of acknowledgement of the original source, and a payment of $15 per page, table or figure of reproduced material. -
Systemic Contact Dermatitis Dianne L
ALLERGEN FOCUS SYSTEMIC CONTACT DERMATITIS DIANNE L. SILVESTRI, MD in time, even to something that the patient has been using regularly for a short period of time or intermit- tently for years. In certain cases, other related disorders such as irritant con- tact dermatitis (ICD) and contact ur- ticaria (CU) may be relevant; history, rather than patch testing, can point to these as the correct diagnosis for the patient. It is important to note that ICD, the most prevalent form of con- tact dermatitis, can, at times, precede or occur concomitantly with ACD.4,5 Unlike ACD, ICD is not immune- mediated. It occurs secondary to contact with an irritating or abrasive substance. CU, on the other hand, represents the least prevalent form of ICD. The wheal and flare reaction of CU is an IgE- and mast cell-mediated immune phenomenon of immediate- type hypersensitivity. Although this form of contact reaction is rare, it is important to recognize because of its potential to produce serious anaphy- lactic-type reactions.6–8 This column highlights ACD, focus- ing on significant allergens, regional llergic Con- to comply with allergen avoidance are presentations of dermatitis and top- tact Dermati- at risk for recurrent or sustained der- ic-based allergic manifestations and tis (ACD) is an matitis or progression to a systematized offers clinical tips for diagnosis and A 2,3 important disease that presentation. In fact, patient education treatment. This month, we feature an affects 14.5 million often begins before the diagnostic patch uncommon but especially important Americans each year.1 tests are ever placed, to ensure that ACD category of allergic dermatitis — sys- Dianne L.