Presenters: Philip R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hyperhidrosis: Sweating out the Details
Focus on CME at the Université de Montréal Hyperhidrosis: Sweating Out the Details Antranik Benohanian, MD, FRCPC; and Nowell Solish, MD, FRCPC Presented at the 250th meeting of the Montreal Dermatological Society, April 2003 yperhidrosis (HH) remains a relatively Table 1 Hunknown disorder to the general public Most commonly affected sites and health-care professionals. According to the literature, 0.5% to 1% of the population is Site Prevalence affected by HH. However, a recent survey held Facial 68.9% in the U.S. places that figure at 2.8%; thus, Axillary 50.8% revealing that the prevalence is underrated. Plantar 28.7% Among those affected, only 38% had discussed Palmar 24.8% the problem with a health professional.1 HH may be classified as primary or sec- ondary; either type can be localized or gen- Besides affecting quality of life, HH predis- eralized. Table 1 lists the most commonly poses its victims to a host of dermatologic dis- affected sites. orders (Table 2).3 The control of HH would also control the associated disease condition, as has Impact on quality of life been recently reported with the treatment of dyshidrotic hand dermatitis with intradermal HH is known to be a socially embarrassing and botulinum toxin.4 occupationally disabling disorder. Many patients suffer in silence. Figure 1 illustrates the How is HH treated? impact HH has on quality of life.2 Those with axillary HH often have to change Systemic approach clothing several times a day and throw out Minor sedatives, such as amitriptyline and clothing because of the damage caused to fabric hydroxyzine, produce an anticholinergic, as and leather. -
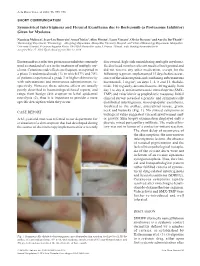
Symmetrical Intertriginous and Flexural Exanthema Due to Bortezomib (A Proteasome Inhibitor) Given for Myeloma
Acta Derm Venereol 2016; 96: 995–996 SHORT COMMUNICATION Symmetrical Intertriginous and Flexural Exanthema due to Bortezomib (a Proteasome Inhibitor) Given for Myeloma Nausicaa Malissen1, Jean-Luc Bourrain1, Anca Chiriac2, Aline Montet1, Laure Vincent3, Olivier Dereure1 and Aurélie Du-Thanh1* 1Dermatology Department, 2Pneumology – Allergology Department, Montpellier University Hospital, and 3Clinical Hematology Department, Montpellier University Hospital, 80 avenue Augustin Fliche, FR-34295 Montpellier cedex 5, France. *E-mail: [email protected] Accepted Mar 17, 2016; Epub ahead of print Mar 22, 2016 Bortezomib is a selective proteasome inhibitor currently discovered, high-risk smouldering multiple myeloma. used as standard of care in the treatment of multiple my- He disclosed no other relevant medical background and eloma. Cutaneous side-effects are frequent, as reported in did not receive any other medication, except for the a phase 3 randomized study (1), in which 57% and 70% following regimen, implemented 15 days before occur- of patients experienced a grade 3 or higher skin toxicity rence of the skin eruption and combining subcutaneous with subcutaneous and intravenous administration, re- bortezomib, 1 mg/m2, on days 1, 4, 8 and 11, thalido- spectively. However, these adverse effects are usually mide, 100 mg daily, dexamethasone, 40 mg daily from poorly described in haematological-based reports, and day 1 to day 4, sulfamethoxazole trimethoprim (SMX- range from benign skin eruption to lethal epidermal TMP) and valaciclovir as prophylactic measures. Initial necrolysis (2), thus it is important to provide a more clinical survey revealed a pruritic and symmetrically specific description when they occur. distributed intertriginous, maculopapular exanthema, localized to the axillae, antecubital fossae, groin, neck and buttocks (Fig. -

Fundamentals of Dermatology Describing Rashes and Lesions
Dermatology for the Non-Dermatologist May 30 – June 3, 2018 - 1 - Fundamentals of Dermatology Describing Rashes and Lesions History remains ESSENTIAL to establish diagnosis – duration, treatments, prior history of skin conditions, drug use, systemic illness, etc., etc. Historical characteristics of lesions and rashes are also key elements of the description. Painful vs. painless? Pruritic? Burning sensation? Key descriptive elements – 1- definition and morphology of the lesion, 2- location and the extent of the disease. DEFINITIONS: Atrophy: Thinning of the epidermis and/or dermis causing a shiny appearance or fine wrinkling and/or depression of the skin (common causes: steroids, sudden weight gain, “stretch marks”) Bulla: Circumscribed superficial collection of fluid below or within the epidermis > 5mm (if <5mm vesicle), may be formed by the coalescence of vesicles (blister) Burrow: A linear, “threadlike” elevation of the skin, typically a few millimeters long. (scabies) Comedo: A plugged sebaceous follicle, such as closed (whitehead) & open comedones (blackhead) in acne Crust: Dried residue of serum, blood or pus (scab) Cyst: A circumscribed, usually slightly compressible, round, walled lesion, below the epidermis, may be filled with fluid or semi-solid material (sebaceous cyst, cystic acne) Dermatitis: nonspecific term for inflammation of the skin (many possible causes); may be a specific condition, e.g. atopic dermatitis Eczema: a generic term for acute or chronic inflammatory conditions of the skin. Typically appears erythematous, -

Symmetrical Drug-Related Intertriginous and Flexural Exanthema Secondary to Topical 5-Fluorouracil
Symmetrical Drug-Related Intertriginous and Flexural Exanthema Secondary to Topical 5-Fluorouracil Roxann Powers, MD; Rachel Gordon, MD; Kenrick Roberts, MD; Rodney Kovach, MD We report the case of a 56-year-old man who fossae and axillae. His dermatologist stopped the developed a distinctive skin eruption after treat- 5-FU and started prednisone, erythromycin, hydro- ing actinic keratoses on the dorsal aspects of cortisone ointment, and fexofenadine hydrochloride. his right and left hands with topical 5-fluorouracil Over the next week, the areas became eroded and the (5-FU). The distribution of his rash was charac- pain worsened. A biopsy was performed. The hydro- teristic of symmetrical drug-related intertriginous cortisone was discontinued and he was referred for a and flexural exanthema (SDRIFE), also known as second opinion. baboon syndrome. Medications at presentation included erythromycin, Cutis. 2012;89:225-228. fexofenadine hydrochloride, acetaminophen-codeine, CUTISprednisone, petrolatum ointment, metformin Case Report hydrochloride, enalapril maleate, ranitidine hydro- A 56-year-old man with a history of diabetes mel- chloride, simvastatin, triamterene/hydrochlorothiazide, litus, hypertension, hyperlipidemia, reflux, squamous aspirin, and omeprazole. He reported no cell carcinoma in situ of his right hand, and actinic known allergies. keratoses of the right Doand left hands presentedNot with a PhysicalCopy examination revealed an overweight painful, burning, pruritic, erythematous, and blister- man who appeared agitated and reported substantial ing rash on his medial thighs, scrotum, penis, antecu- discomfort. He had well-demarcated, violaceous, red bital fossae, axillae, and dorsal hands. The patient had erosions on his medial thighs (Figure 1), scrotum, a successful history of treatment of actinic keratosis and penis; erythematous denuded patches in the on his right hand with topical 5-fluorouracil (5-FU) antecubital fossae (Figure 2) and axillae; and crusty 8 years prior to presentation. -

“The Red Face” and More Clinical Pearls
“The Red Face” and More Clinical Pearls Courtney R. Schadt, MD, FAAD Assistant Professor Residency Program Director University of Louisville Associates in Dermatology I have no disclosures or conflicts of interest Part 1: The Red Face: Objectives • Distinguish and diagnose common eruptions of the face • Recognize those with potential implications for internal disease • Learn basic treatment options Which patient(s) has an increased risk of hypertension and hyperlipidemia? A B C Which patient(s) has an increased risk of hypertension and hyperlipidemia? A Seborrheic Dermatitis B C Psoriasis Seborrheic Dermatitis Goodheart HP. Goodheart's photoguide of common skin disorders, 2nd ed, Lippincott Williams & Wilkins, Philadelphia 2003. Copyright © 2003 Lippincott Williams & Wilkins. Seborrheic Dermatitis • Erythematous scaly eruption • Infants= “Cradle Cap” • Reappear in adolescence or later in life • Chronic, remissions and flares; worse with stress, cold weather • Occurs on areas of body with increased sebaceous glands • Unclear role of Malassezia; could be immune response; no evidence of overgrowth Seborrheic Dermatitis Severe Seb Derm: THINK: • HIV (can also be more diffuse on trunk) • Parkinson’s (seb derm improves with L-dopa therapy) • Other neurologic disorders • Neuroleptic agents • Unclear etiology 5MinuteClinicalConsult Clinical Exam • Erythema/fine scale • Scalp • Ears • Nasolabial folds • Beard/hair bearing areas Goodheart HP. Goodheart's photoguide of common skin disorders, 2nd ed, Lippincott • Ill-defined Williams & Wilkins, Philadelphia -

Therapies for Common Cutaneous Fungal Infections
MedicineToday 2014; 15(6): 35-47 PEER REVIEWED FEATURE 2 CPD POINTS Therapies for common cutaneous fungal infections KENG-EE THAI MB BS(Hons), BMedSci(Hons), FACD Key points A practical approach to the diagnosis and treatment of common fungal • Fungal infection should infections of the skin and hair is provided. Topical antifungal therapies always be in the differential are effective and usually used as first-line therapy, with oral antifungals diagnosis of any scaly rash. being saved for recalcitrant infections. Treatment should be for several • Topical antifungal agents are typically adequate treatment weeks at least. for simple tinea. • Oral antifungal therapy may inea and yeast infections are among the dermatophytoses (tinea) and yeast infections be required for extensive most common diagnoses found in general and their differential diagnoses and treatments disease, fungal folliculitis and practice and dermatology. Although are then discussed (Table). tinea involving the face, hair- antifungal therapies are effective in these bearing areas, palms and T infections, an accurate diagnosis is required to ANTIFUNGAL THERAPIES soles. avoid misuse of these or other topical agents. Topical antifungal preparations are the most • Tinea should be suspected if Furthermore, subsequent active prevention is commonly prescribed agents for dermatomy- there is unilateral hand just as important as the initial treatment of the coses, with systemic agents being used for dermatitis and rash on both fungal infection. complex, widespread tinea or when topical agents feet – ‘one hand and two feet’ This article provides a practical approach fail for tinea or yeast infections. The pharmacol- involvement. to antifungal therapy for common fungal infec- ogy of the systemic agents is discussed first here. -

Not Just Luck
EDITORIAL Not Just Luck few months ago, I described a 52-year-old and encourage potential authors and reviewers. At a female patient’s puzzling rash to a colleague: memorable meeting last winter, during a howling snow- a striking symmetrical, bright-red, sharply- storm one dark Thursday night, I met Susan Busch and defined macular erythema of the genital/ Jane Tallent, the 2009Y2010 Lahey Clinic Dermatology inguinal and medial-gluteal areas, and much Nurse Practitioner Fellowship fellows, quintessential ex- less prominently, the axillae and inframmary folds. Sitting amples of individuals who heartily seek out learning op- Aon her desk was an article, just read, describing this un- portunities. We talked animatedly about ways they might usual drug eruption. Coined the Bbaboon syndrome[ be- contribute to the Journal, and I forgot how sweet it would cause of the resemblance to the red buttocks of baboons, it have been to stay home and light a fire in the fireplace. has been renamed the less colorful, less memorable, but less- When an uncanny opportunity presents itself to apply some- unnerving-to-be-diagnosed- thing we have just learned, we can remind ourselves that we with Bsymmetrical drug-related helped create this fortunate situation; we helped create our intertriginous and flexural exan- own luck. A dermatology nurse approached me at the 2009 thema[ (SDRIFE; Hausermann, Dermatology Nurses’ Association summer meeting in Boston, Harr, & Bircher, 2004). reporting that only a day or two after she was introduced to I said it was good luck that Merkel cell carcinoma by the JDNA article by Victoria she had just read this article, and Garcia-Albea (nee Beebe; Beebe, 2009), she heard colleagues a colleague commented, para- discussing a new patient diagnosed with Merkel cell cancer in phrasing Louis Pasteur, BChance their practice. -

Clinical Communications Symmetrical Drug
Clinical Communications Symmetrical drug-related intertriginous and flexural exanthema: A little-known drug allergy Tullia De Risi-Pugliese, Héloïse Barailler, Aurore Hamelin, Emmanuelle Amsler, Hafida Gaouar, Flore Kurihara, Marie Laure Jullie, Eric Dean Merrill, Annick Barbaud, Philippe Moguelet, et al. To cite this version: Tullia De Risi-Pugliese, Héloïse Barailler, Aurore Hamelin, Emmanuelle Amsler, Hafida Gaouar, et al.. Clinical Communications Symmetrical drug-related intertriginous and flexural exanthema: A little- known drug allergy. Journal of Allergy and Clinical Immunology: In Practice, Elsevier, 2020, 8 (9), pp.3185-3189.e4. 10.1016/j.jaip.2020.04.052. hal-02995700 HAL Id: hal-02995700 https://hal.sorbonne-universite.fr/hal-02995700 Submitted on 9 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Clinical Communications Symmetrical drug-related intertriginous 2-7) large or small skin folds (especially inguinal, gluteal, axillary, and flexural exanthema: A little-known and mammary) (Figure 1, Table I) were observed. Four patients drug allergy had localized skin vesicles or bullae and 1 (no. 16) had mucosal Tullia de Risi-Pugliese, MDa,b, Héloïse Barailler, MDc, involvement. The eruption occurred shortly after drug exposure a a,b (median, 22 hours; mean, 34; range, 0.5-120). -

Or Moisture-Associated Skin Damage, Due to Perspiration: Expert Consensus on Best Practice
A Practical Approach to the Prevention and Management of Intertrigo, or Moisture-associated Skin Damage, due to Perspiration: Expert Consensus on Best Practice Consensus panel R. Gary Sibbald MD Professor, Medicine and Public Health University of Toronto Toronto, ON Judith Kelley RN, BSN, CWON Henry Ford Hospital – Main Campus Detroit, MI Karen Lou Kennedy-Evans RN, FNP, APRN-BC KL Kennedy LLC Tucson, AZ Chantal Labrecque RN, BSN, MSN CliniConseil Inc. Montreal, QC Nicola Waters RN, MSc, PhD(c) Assistant Professor, Nursing Mount Royal University A supplement of Calgary, AB The development of this consensus document has been supported by Coloplast. Editorial support was provided by Joanna Gorski of Prescriptum Health Care Communications Inc. This supplement is published by Wound Care Canada and is available at www.woundcarecanada.ca. All rights reserved. Contents may not be reproduced without written permission of the Canadian Association of Wound Care. © 2013. 2 Wound Care Canada – Supplement Volume 11, Number 2 · Fall 2013 Contents Introduction ................................................................... 4 Complications of Intertrigo ......................................11 Moisture-associated skin damage Secondary skin infection ...................................11 and intertrigo ................................................................. 4 Organisms in intertrigo ..............................11 Consensus Statements ................................................ 5 Specific types of infection .................................11 -

Skin Allergy to Azole Antifungal Agents for Systemic Use: a Review of the Literature
Send Orders for Reprints to [email protected] Recent Patents on Inflammation & Allergy Drug Discovery 2019, 13, 1-14 1 REVIEW ARTICLE Skin Allergy to Azole Antifungal Agents for Systemic Use: A Review of the Literature Gianfranco Calogiuri1,3, Lene H. Garvey2, Eustachio Nettis3, Paolo Romita4, Elisabetta Di Leo5, Riccardo Caruso6, Lavjay Butani7 and Caterina Foti4,* 1Department of Pneumology and Allergy Hospital Sacro Cuore - Gallipoli, Lecce, Italy; 2Allergy Clinic, Department of Dermato-Allergology, Herlev and Gentofte Hospital, University of Copenhagen, Hellerup, Denmark; 3Department of Biomedical Sciences and Human Oncology Unit of Internal Medicine "G. Baccelli", "Aldo Moro" University of Bari Medical School, Policlinico, Bari, Italy; 4Department of Biomedical Science and Human Oncology, Dermatological Clinic, University of Bari, Bari, Italy; 5Section of Allergy and Clinical Immunology, Unit of Internal Medicine-"F. Miulli" Hospital, Acquaviva delle Fonti, Bari, Italy; 6Clinica Salus - Brindisi, Brindisi, Italy; 7Section of Pediatric Nephrology, Department of Pediatrics, University of California Davis Medical Center, Sacramento, CA, USA Abstract: Background: Antifungal azoles are the first-line agents used to treat topical and, above all, systemic mycosis. The latter could be life-threating infections in immunocompromised patients. Che- motherapeutic antibiotics, including antifungal azoles, may induce hypersensitivity reactions; however, such immunologic adverse reactions have not been defined and carefully investigated. Objective: The study aimed to provide an update on the evaluation and diagnosis of skin allergy to azole antifungal agents. Methods: This is a systematic review performed on PubMed and Google Schoolbarusing using the key terms “allergy, hypersensitivity, anaphylaxis, immediate-type reaction, delayed-type reaction, keto- conazole, fluconazole, posaconazole, voriconazole, itraconazole, triazoles, imidazoles, antifungals, A R T I C L E H I S T O R Y antimycotics”. -

General Aspects 14 Niels K
14_199_254* 05.11.2005 10:15 Uhr Seite 201 Chapter 14 General Aspects 14 Niels K. Veien Contents 14.1 Introduction . 201 14.4.2.1 Cement Ulcerations . 224 14.4.2.2 Pigmented Contact Dermatitis . 225 14.2 The Medical History of the Patient . 202 14.4.2.3 Caterpillar Dermatitis and Irritant Dermatitis 14.2.1 History of Hereditary Diseases . 202 from Plants and Animals . 225 14.2.2 General Medical History . 202 14.4.2.4 Head and Neck Dermatitis . 225 14.2.3 History of Previous Dermatitis . 203 14.4.2.5 Dermatitis from Transcutaneous Delivery 14.2.4 Time of Onset . 203 Systems . 225 14.2.5 History of Aggravating Factors . 204 14.4.2.6 Berloque Dermatitis . 226 14.2.6 Course of the Dermatitis . 205 14.4.2.7 Stomatitis due to Mercury or Gold Allergy . 226 14.2.7 Types of Symptoms . 205 14.5 Regional Contact Dermatitis . 226 14.3 Clinical Features of Eczematous Reactions . 206 14.5.1 Dermatitis of the Scalp . 226 14.3.1 Acute and Recurrent Dermatitis . 206 14.5.2 Dermatitis of the Face and Neck . 228 14.3.2 Chronic Dermatitis . 210 14.5.2.1 The Lips . 230 14.3.3 Nummular (Discoid) Eczema . 211 14.5.2.2 The Eyes and Eyelids . 230 14.3.4 Secondarily Infected Dermatitis . 211 14.5.2.3 The Ear . 231 14.3.5 Clinical Features of Contact Dermatitis 14.5.3 Dermatitis of the Trunk . 232 in Specific Groups of Persons . 212 14.5.3.1 The Axillary Region . -

Diaper Dermatitis in Infant Skin: Causes and Mitigation
Diaper Dermatitis in Infant Skin: Causes and Mitigation Josh Gregorio, PhD, and Karien Rodriguez, PhD Introduction Classifications of Diaper Dermatitis Infants under the age of two, especially preterm neonates, are Diaper dermatitis can be classified as mild, moderate, or vulnerable to developing skin irritation in the diapered region. severe, and is dependent on skin involvement and the degree Overhydration or prolonged skin contact with urine and feces of inflammation (Figure 1). Characteristics of mild diaper can result in breakdown of the skin barrier (the protective outer dermatitis include shiny erythema with or without scales, layer of the skin), leading to irritation and the appearance of a whereas more severe cases have intense erythema, ulcerations, rash. This event is known as diaper rash or diaper dermatitis, and pustule and vesicle eruptions. general terms describing skin inflammation in the diaper region. A B Diaper dermatitis is among the most common skin disorders of infancy. It accounts for 10-20% of all skin disorders treated by pediatricians and the highest incidence occurs in children between 9 and 12 months of age.1,2 If left untreated, progressive skin irritation in the diapered region can lead to secondary infections, including Candida albicans (candida dermatosis) and bacterial infections, that require additional treatment by a C D physician. Types of Diaper Dermatitis Although there are many types of diaper dermatoses (Table 1), most incidences arise from a nonallergic rash resulting from chemical, physical, or mechanical irritation called irritant contact dermatitis. Figure 1. Representative images of diaper dermatitis severity range: (A) healthy skin, (B) slight, (C) mild, (D) severe.