Contact Dermatitis: a Practice Parametereupdate 2015
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Contact Vitiligo Following Allergic Contact Dermatitis *Ricardo Ruiz-Villaverde, Francisco J Navarro-Triviño
SUBMITTED 19 JAN 21 REVISION REQ. 17 MAR 21; REVISION 5 APR 21 ACCEPTED 21 APR 21 ONLINE-FIRST: MAY 2021 DOI: https://doi.org/10.18295/squmj.5.2021.078 Contact Vitiligo Following Allergic Contact Dermatitis *Ricardo Ruiz-Villaverde, Francisco J Navarro-Triviño Department of Dermatology, Hospital Universitario San Cecilio, Granada, Spain *Corresponding Author’s e-mail: [email protected] Introduction A 45-year-old man, construction worker, with no personal history of psoriasis, atopic dermatitis, and vitiligo, was referred to our Contact Eczema Department with a chronic hand eczema and skin depigmentation over a period of 12 months. Skin depigmentation appeared few months later regarding the primary eczema. The patient reported the use of rubber gloves for many years. He had noticed itching and mild erythema over both hands. Currently, he wears nitrile gloves at work. Physical examination showed symmetric erythematous-squamous, hyperkeratotic and fissured plaques on both hands (Fig. 1A), and ventral aspect of wrists (Fig. 1B). Skin depigmentation areas showed irregular edges (Fig. 1C). Wood´s lamp examination accentuated the depigmentation areas overlap the eczema (Fig. 2A-B), without vitiligo pattern. No other anatomical sites were involved. Blood test showed no significant alterations, including data from autoimmune thyroiditis, celiac disease, and pernicious anaemia. Patch tests were performed with the European Comprehensive Baseline Series (Chemotechnique Diagnostics, Vellinge, Sweden), rubber additives series (Chemotechnique Diagnostics), and hydroquinone monobenzylether 1% pet (Shoe series, Chemotechnique Diagnosis). The results were interpreted according to the criteria of the International Contact Dermatitis Research Group. Patch tests were read on day (D) 2 and D4. -

(12) Patent Application Publication (10) Pub. No.: US 2008/0317805 A1 Mckay Et Al
US 20080317805A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0317805 A1 McKay et al. (43) Pub. Date: Dec. 25, 2008 (54) LOCALLY ADMINISTRATED LOW DOSES Publication Classification OF CORTICOSTEROIDS (51) Int. Cl. A6II 3/566 (2006.01) (76) Inventors: William F. McKay, Memphis, TN A6II 3/56 (2006.01) (US); John Myers Zanella, A6IR 9/00 (2006.01) Cordova, TN (US); Christopher M. A6IP 25/04 (2006.01) Hobot, Tonka Bay, MN (US) (52) U.S. Cl. .......... 424/422:514/169; 514/179; 514/180 (57) ABSTRACT Correspondence Address: This invention provides for using a locally delivered low dose Medtronic Spinal and Biologics of a corticosteroid to treat pain caused by any inflammatory Attn: Noreen Johnson - IP Legal Department disease including sciatica, herniated disc, Stenosis, mylopa 2600 Sofamor Danek Drive thy, low back pain, facet pain, osteoarthritis, rheumatoid Memphis, TN38132 (US) arthritis, osteolysis, tendonitis, carpal tunnel syndrome, or tarsal tunnel syndrome. More specifically, a locally delivered low dose of a corticosteroid can be released into the epidural (21) Appl. No.: 11/765,040 space, perineural space, or the foramenal space at or near the site of a patient's pain by a drug pump or a biodegradable drug (22) Filed: Jun. 19, 2007 depot. E Day 7 8 Day 14 El Day 21 3OO 2OO OO OO Control Dexamethasone DexamethasOne Dexamethasone Fuocinolone Fluocinolone Fuocinolone 2.0 ng/hr 1Ong/hr 50 ng/hr 0.0032ng/hr 0.016 ng/hr 0.08 ng/hr Patent Application Publication Dec. 25, 2008 Sheet 1 of 2 US 2008/0317805 A1 900 ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 80.0 - 7OO – 6OO - 5OO - E Day 7 EDay 14 40.0 - : El Day 21 2OO - OO = OO – Dexamethasone Dexamethasone Dexamethasone Fuocinolone Fluocinolone Fuocinolone 2.0 ng/hr 1Ong/hr 50 ng/hr O.OO32ng/hr O.016 ng/hr 0.08 nghr Patent Application Publication Dec. -

Antiseptics and Disinfectants for the Treatment Of
Verstraelen et al. BMC Infectious Diseases 2012, 12:148 http://www.biomedcentral.com/1471-2334/12/148 RESEARCH ARTICLE Open Access Antiseptics and disinfectants for the treatment of bacterial vaginosis: A systematic review Hans Verstraelen1*, Rita Verhelst2, Kristien Roelens1 and Marleen Temmerman1,2 Abstract Background: The study objective was to assess the available data on efficacy and tolerability of antiseptics and disinfectants in treating bacterial vaginosis (BV). Methods: A systematic search was conducted by consulting PubMed (1966-2010), CINAHL (1982-2010), IPA (1970- 2010), and the Cochrane CENTRAL databases. Clinical trials were searched for by the generic names of all antiseptics and disinfectants listed in the Anatomical Therapeutic Chemical (ATC) Classification System under the code D08A. Clinical trials were considered eligible if the efficacy of antiseptics and disinfectants in the treatment of BV was assessed in comparison to placebo or standard antibiotic treatment with metronidazole or clindamycin and if diagnosis of BV relied on standard criteria such as Amsel’s and Nugent’s criteria. Results: A total of 262 articles were found, of which 15 reports on clinical trials were assessed. Of these, four randomised controlled trials (RCTs) were withheld from analysis. Reasons for exclusion were primarily the lack of standard criteria to diagnose BV or to assess cure, and control treatment not involving placebo or standard antibiotic treatment. Risk of bias for the included studies was assessed with the Cochrane Collaboration’s tool for assessing risk of bias. Three studies showed non-inferiority of chlorhexidine and polyhexamethylene biguanide compared to metronidazole or clindamycin. One RCT found that a single vaginal douche with hydrogen peroxide was slightly, though significantly less effective than a single oral dose of metronidazole. -

Commission Decision of 8 February 2010 Concerning The
L 36/36 EN Official Journal of the European Union 9.2.2010 COMMISSION DECISION of 8 February 2010 concerning the non-inclusion of certain substances in Annex I, IA or IB to Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market (notified under document C(2010) 751) (Text with EEA relevance) (2010/72/EU) THE EUROPEAN COMMISSION, concerned should therefore not be included in Annex I, IA or IB to Directive 98/8/EC. Having regard to the Treaty on the Functioning of the European Union, (6) In the interest of legal certainty, biocidal products Having regard to Directive 98/8/EC of the European Parliament containing active substances for the product-types and of the Council of 16 February 1998 concerning the placing indicated in the Annex to this Decision should no of biocidal products on the market ( 1), and in particular the longer be placed on the market, with effect from a second subparagraph of Article 16(2) thereof, specific date. Whereas: (7) The measures provided for in this Decision are in accordance with the opinion of the Standing (1) Commission Regulation (EC) No 1451/2007 of Committee on Biocidal Products, 4 December 2007 on the second phase of the 10-year work programme referred to in Article 16(2) of Directive HAS ADOPTED THIS DECISION: 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the Article 1 market ( 2) establishes a list of active substances to be assessed, with a view to their possible inclusion in The substances indicated in the Annex to this Decision shall not Annex I, IA or IB to Directive 98/8/EC. -

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Seborrheic Dermatitis: an Overview ROBERT A
Seborrheic Dermatitis: An Overview ROBERT A. SCHWARTZ, M.D., M.P.H., CHRISTOPHER A. JANUSZ, M.D., and CAMILA K. JANNIGER, M.D. University of Medicine and Dentistry at New Jersey-New Jersey Medical School, Newark, New Jersey Seborrheic dermatitis affects the scalp, central face, and anterior chest. In adolescents and adults, it often presents as scalp scaling (dandruff). Seborrheic dermatitis also may cause mild to marked erythema of the nasolabial fold, often with scaling. Stress can cause flare-ups. The scales are greasy, not dry, as commonly thought. An uncommon generalized form in infants may be linked to immunodeficiencies. Topical therapy primarily consists of antifungal agents and low-potency steroids. New topical calcineurin inhibitors (immunomodulators) sometimes are administered. (Am Fam Physician 2006;74:125-30. Copyright © 2006 American Academy of Family Physicians.) eborrheic dermatitis can affect patients levels, fungal infections, nutritional deficits, from infancy to old age.1-3 The con- neurogenic factors) are associated with the dition most commonly occurs in condition. The possible hormonal link may infants within the first three months explain why the condition appears in infancy, S of life and in adults at 30 to 60 years of age. In disappears spontaneously, then reappears adolescents and adults, it usually presents as more prominently after puberty. A more scalp scaling (dandruff) or as mild to marked causal link seems to exist between seborrheic erythema of the nasolabial fold during times dermatitis and the proliferation of Malassezia of stress or sleep deprivation. The latter type species (e.g., Malassezia furfur, Malassezia tends to affect men more often than women ovalis) found in normal dimorphic human and often is precipitated by emotional stress. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group D07A (Corticosteroids, Plain) Table of Contents Page INTRODUCTION 4 DISCLAIMER 6 GLOSSARY OF TERMS USED IN THIS DOCUMENT 7 ACTIVE SUBSTANCES Methylprednisolone (ATC: D07AA01) 8 Hydrocortisone (ATC: D07AA02) 9 Prednisolone (ATC: D07AA03) 11 Clobetasone (ATC: D07AB01) 13 Hydrocortisone butyrate (ATC: D07AB02) 16 Flumetasone (ATC: D07AB03) 18 Fluocortin (ATC: D07AB04) 21 Fluperolone (ATC: D07AB05) 22 Fluorometholone (ATC: D07AB06) 23 Fluprednidene (ATC: D07AB07) 24 Desonide (ATC: D07AB08) 25 Triamcinolone (ATC: D07AB09) 27 Alclometasone (ATC: D07AB10) 29 Hydrocortisone buteprate (ATC: D07AB11) 31 Dexamethasone (ATC: D07AB19) 32 Clocortolone (ATC: D07AB21) 34 Combinations of Corticosteroids (ATC: D07AB30) 35 Betamethasone (ATC: D07AC01) 36 Fluclorolone (ATC: D07AC02) 39 Desoximetasone (ATC: D07AC03) 40 Fluocinolone Acetonide (ATC: D07AC04) 43 Fluocortolone (ATC: D07AC05) 46 2 Diflucortolone (ATC: D07AC06) 47 Fludroxycortide (ATC: D07AC07) 50 Fluocinonide (ATC: D07AC08) 51 Budesonide (ATC: D07AC09) 54 Diflorasone (ATC: D07AC10) 55 Amcinonide (ATC: D07AC11) 56 Halometasone (ATC: D07AC12) 57 Mometasone (ATC: D07AC13) 58 Methylprednisolone Aceponate (ATC: D07AC14) 62 Beclometasone (ATC: D07AC15) 65 Hydrocortisone Aceponate (ATC: D07AC16) 68 Fluticasone (ATC: D07AC17) 69 Prednicarbate (ATC: D07AC18) 73 Difluprednate (ATC: D07AC19) 76 Ulobetasol (ATC: D07AC21) 77 Clobetasol (ATC: D07AD01) 78 Halcinonide (ATC: D07AD02) 81 LIST OF AUTHORS 82 3 INTRODUCTION The availability of medicines with or without a medical prescription has implications on patient safety, accessibility of medicines to patients and responsible management of healthcare expenditure. The decision on prescription status and related supply conditions is a core competency of national health authorities. -

Scalp Eczema Factsheet the Scalp Is an Area of the Body That Can Be Affected by Several Types of Eczema
12 Scalp eczema factsheet The scalp is an area of the body that can be affected by several types of eczema. The scalp may be dry, itchy and scaly in a chronic phase and inflamed (red), weepy and painful in an acute (eczema flare) phase. Aside from eczema, there are a number of reasons why the scalp can become dry and itchy (e.g. psoriasis, fungal infection, ringworm, head lice etc.), so it is wise to get a firm diagnosis if there is uncertainty. Types of eczema • Hair clips and headgear – especially those containing that affect the scalp rubber or nickel. Seborrhoeic eczema (dermatitis) is one of the most See the NES booklet on Contact Dermatitis for more common types of eczema seen on the scalp and hairline. details. It can affect babies (cradle cap), children and adults. The Irritant contact dermatitis is a type of eczema that skin appears red and scaly and there is often dandruff as occurs when the skin’s surface is irritated by a substance well, which can vary in severity. There may also be a rash that causes the skin to become dry, red and itchy. on other parts of the face, such as around the eyebrows, For example, shampoos, mousses, hair gels, hair spray, eyelids and sides of the nose. Seborrhoeic eczema can perm solution and fragrance can all cause irritant contact become infected. See the NES factsheets on Adult dermatitis. See the NES booklet on Contact Dermatitis for Seborrhoeic Dermatitis and Infantile Seborrhoeic more details. Dermatitis and Cradle Cap for more details. -

Pompholyx Factsheet Pompholyx Eczema (Also Known As Dyshidrotic Eczema/Dermatitis) Is a Type of Eczema That Usually Affects the Hands and Feet
12 Pompholyx factsheet Pompholyx eczema (also known as dyshidrotic eczema/dermatitis) is a type of eczema that usually affects the hands and feet. In most cases, pompholyx eczema involves the development of intensely itchy, watery blisters, mostly affecting the sides of the fingers, the palms of the hands and soles of the feet. Some people have pompholyx eczema on their hands and/or feet with other types of eczema elsewhere on the body. This condition can occur at any age but is usually seen in adults under 40, and is more common in women. The skin is initially very itchy with a burning sensation of heat and prickling in the palms and/or soles. Then comes a sudden crop of small blisters (vesicles), which turn into bigger weepy blisters, which can become infected, causing redness, pain, swelling and pustules. There is often subsequent peeling as the skin dries out, and then the skin can become red and dry with painful cracks (skin fissures). Pompholyx eczema can also affect the nail folds and skin around the nails, causing swelling (paronychia). What causes it? A reaction could be the result of contact with potential irritants such as soap, detergents, solvents, acids/alkalis, The exact causes of pompholyx eczema are not known, chemicals and soil, causing irritant contact dermatitis. Or although it is thought that factors such as stress, there could be an allergic reaction to a substance that is sensitivity to metal compounds (such as nickel, cobalt or not commonly regarded as an irritant, such as rubber or chromate), heat and sweating can aggravate this nickel, causing allergic contact dermatitis. -

A New Robust Technique for Testing of Glucocorticosteroids in Dogs and Horses Terry E
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2007 A new robust technique for testing of glucocorticosteroids in dogs and horses Terry E. Webster Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Veterinary Toxicology and Pharmacology Commons Recommended Citation Webster, Terry E., "A new robust technique for testing of glucocorticosteroids in dogs and horses" (2007). Retrospective Theses and Dissertations. 15029. https://lib.dr.iastate.edu/rtd/15029 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. A new robust technique for testing of glucocorticosteroids in dogs and horses by Terry E. Webster A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Toxicology Program o f Study Committee: Walter G. Hyde, Major Professor Steve Ensley Thomas Isenhart Iowa State University Ames, Iowa 2007 Copyright © Terry Edward Webster, 2007. All rights reserved UMI Number: 1446027 Copyright 2007 by Webster, Terry E. All rights reserved. UMI Microform 1446027 Copyright 2007 by ProQuest Information and Learning Company. All rights reserved. This microform edition is protected against unauthorized copying under Title 17, United States Code. ProQuest Information and Learning Company 300 North Zeeb Road P.O. Box 1346 Ann Arbor, MI 48106-1346 ii DEDICATION I want to dedicate this project to my wife, Jackie, and my children, Shauna, Luke and Jake for their patience and understanding without which this project would not have been possible. -

1532 Corticosteroids
1532 Corticosteroids olone; Ultraderm; Malaysia: Synalar†; Mex.: Cortifung-S; Cortilona; Gr.: Lidex; Ital.: Flu-21†; Topsyn; Mex.: Topsyn; Norw.: Metosyn; Pharmacopoeias. In Br. Cremisona; Farmacorti; Flumicin; Fluomex; Fusalar; Lonason; Synalar; Philipp.: Lidemol; Lidex; Singapore: Lidex†; Spain: Klariderm†; Novoter; BP 2008 (Fluocortolone Hexanoate). A white or creamy-white, Norw.: Synalar; NZ: Synalar; Philipp.: Aplosyn; Cynozet; Synalar; Syntop- Switz.: To p s y m ; To p s y m i n ; UK: Metosyn; USA: Lidex; Vanos. ic; Pol.: Flucinar; Port.: Oto-Synalar N; Synalar; Rus.: Flucinar (Флуцинар); odourless or almost odourless, crystalline powder. It exhibits pol- Multi-ingredient: Austria: Topsym polyvalent; Ger.: Jelliproct; Topsym ymorphism. Practically insoluble in water and in ether; very Sinaflan (Синафлан); S.Afr.: Cortoderm; Fluoderm; Synalar; Singapore: polyvalent; Hung.: Vipsogal†; Israel: Comagis; Mex.: Topsyn-Y; Philipp.: Flunolone-V; Spain: Co Fluocin Fuerte; Cortiespec; Fluocid Forte; Fluoder- Lidex NGN; Spain: Novoter Gentamicina; Switz.: Mycolog N; Topsym slightly soluble in alcohol and in methyl alcohol; slightly soluble mo Fuerte; Flusolgen; Gelidina; Intradermo Corticosteroi†; Synalar; Synalar polyvalent; UK: Vipsogal. in acetone and in dioxan; sparingly soluble in chloroform. Pro- Rectal Simple; Swed.: Synalar; Switz.: Synalar; Thai.: Cervicum; Flu- ciderm†; Flunolone-V; Fulone; Supralan; Synalar; UK: Synalar; USA: Capex; tect from light. Derma-Smoothe/FS; DermOtic; Fluonid; Flurosyn†; Retisert; Synalar; Syn- emol; Venez.: Bratofil; Fluquinol Simple†; Neo-Synalar; Neoflu†. Fluocortin Butyl (BAN, USAN, rINNM) ⊗ Fluocortolone Pivalate (BANM, rINNM) ⊗ Multi-ingredient: Arg.: Adop-Tar†; Tri-Luma; Austria: Myco-Synalar; Procto-Synalar; Synalar N; Belg.: Procto-Synalar; Synalar Bi-Otic; Braz.: Butil éster de la fluocortina; Butylis Fluocortinas; Fluocortine Fluocortolone, pivalate de; Fluocortolone Trimethylacetate; Dermobel†; Dermoxin; Elotin; Fluo-Vaso; Neocinolon; Otauril†; Otocort†; Butyle; SH-K-203. -
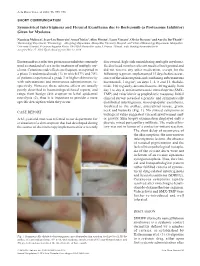
Symmetrical Intertriginous and Flexural Exanthema Due to Bortezomib (A Proteasome Inhibitor) Given for Myeloma
Acta Derm Venereol 2016; 96: 995–996 SHORT COMMUNICATION Symmetrical Intertriginous and Flexural Exanthema due to Bortezomib (a Proteasome Inhibitor) Given for Myeloma Nausicaa Malissen1, Jean-Luc Bourrain1, Anca Chiriac2, Aline Montet1, Laure Vincent3, Olivier Dereure1 and Aurélie Du-Thanh1* 1Dermatology Department, 2Pneumology – Allergology Department, Montpellier University Hospital, and 3Clinical Hematology Department, Montpellier University Hospital, 80 avenue Augustin Fliche, FR-34295 Montpellier cedex 5, France. *E-mail: [email protected] Accepted Mar 17, 2016; Epub ahead of print Mar 22, 2016 Bortezomib is a selective proteasome inhibitor currently discovered, high-risk smouldering multiple myeloma. used as standard of care in the treatment of multiple my- He disclosed no other relevant medical background and eloma. Cutaneous side-effects are frequent, as reported in did not receive any other medication, except for the a phase 3 randomized study (1), in which 57% and 70% following regimen, implemented 15 days before occur- of patients experienced a grade 3 or higher skin toxicity rence of the skin eruption and combining subcutaneous with subcutaneous and intravenous administration, re- bortezomib, 1 mg/m2, on days 1, 4, 8 and 11, thalido- spectively. However, these adverse effects are usually mide, 100 mg daily, dexamethasone, 40 mg daily from poorly described in haematological-based reports, and day 1 to day 4, sulfamethoxazole trimethoprim (SMX- range from benign skin eruption to lethal epidermal TMP) and valaciclovir as prophylactic measures. Initial necrolysis (2), thus it is important to provide a more clinical survey revealed a pruritic and symmetrically specific description when they occur. distributed intertriginous, maculopapular exanthema, localized to the axillae, antecubital fossae, groin, neck and buttocks (Fig.