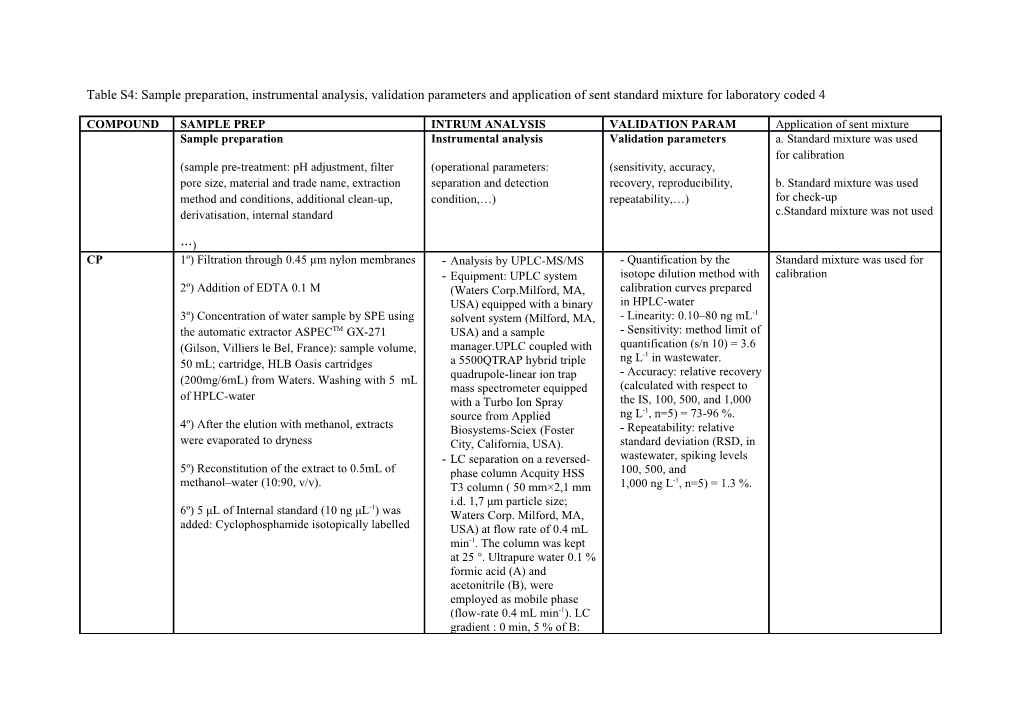
Table S4: Sample preparation, instrumental analysis, validation parameters and application of sent standard mixture for laboratory coded 4
COMPOUND SAMPLE PREP INTRUM ANALYSIS VALIDATION PARAM Application of sent mixture Sample preparation Instrumental analysis Validation parameters a. Standard mixture was used for calibration (sample pre-treatment: pH adjustment, filter (operational parameters: (sensitivity, accuracy, pore size, material and trade name, extraction separation and detection recovery, reproducibility, b. Standard mixture was used method and conditions, additional clean-up, condition,…) repeatability,…) for check-up derivatisation, internal standard c.Standard mixture was not used
…) CP 1º) Filtration through 0.45 µm nylon membranes - Analysis by UPLC-MS/MS - Quantification by the Standard mixture was used for - Equipment: UPLC system isotope dilution method with calibration 2º) Addition of EDTA 0.1 M (Waters Corp.Milford, MA, calibration curves prepared USA) equipped with a binary in HPLC-water 3º) Concentration of water sample by SPE using solvent system (Milford, MA, - Linearity: 0.10–80 ng mL-1 the automatic extractor ASPECTM GX-271 USA) and a sample - Sensitivity: method limit of (Gilson, Villiers le Bel, France): sample volume, manager.UPLC coupled with quantification (s/n 10) = 3.6 ng L-1 in wastewater. 50 mL; cartridge, HLB Oasis cartridges a 5500QTRAP hybrid triple - Accuracy: relative recovery (200mg/6mL) from Waters. Washing with 5 mL quadrupole-linear ion trap mass spectrometer equipped (calculated with respect to of HPLC-water with a Turbo Ion Spray the IS, 100, 500, and 1,000 source from Applied ng L-1, n=5) = 73-96 %. 4º) After the elution with methanol, extracts Biosystems-Sciex (Foster - Repeatability: relative were evaporated to dryness City, California, USA). standard deviation (RSD, in - LC separation on a reversed- wastewater, spiking levels 5º) Reconstitution of the extract to 0.5mL of phase column Acquity HSS 100, 500, and -1 methanol–water (10:90, v/v). T3 column ( 50 mm×2,1 mm 1,000 ng L , n=5) = 1.3 %.
-1 i.d. 1,7 μm particle size; 6º) 5 μL of Internal standard (10 ng μL ) was Waters Corp. Milford, MA, added: Cyclophosphamide isotopically labelled USA) at flow rate of 0.4 mL min-1. The column was kept at 25 °. Ultrapure water 0.1 % formic acid (A) and acetonitrile (B), were employed as mobile phase (flow-rate 0.4 mL min-1). LC gradient : 0 min, 5 % of B: 0.1–4 min, 5-100% B; 4.0– 4.5 min, 100 % B; 4.5–5.0 min, 100–5 % B; 5.0 min, 5 % B during 0.5 min. Sample injection volume 5 μL - MS/MS conditions: positive ESI; SRM quantification transition, 261.1 > 140.0; SRM confirmation transition, 261.1 > 106.0; collision energy, 31 eV and 25 eV, respectively; declustering potential, 101 V.
ETO 1º) Filtration through 0.45 µm nylon membranes - Analysis by UPLC-MS/MS - Quantification by the Standard mixture was used for - Equipment: UPLC system isotope dilution method with calibration 2º) Addition of EDTA 0.1 M (Waters Corp.Milford, MA, calibration curves prepared USA) equipped with a binary in HPLC-water 3º) Concentration of water sample by SPE using solvent system (Milford, MA, - Linearity: 0.10–80 ng mL-1 the automatic extractor ASPECTM GX-271 USA) and a sample - Sensitivity: method limit of (Gilson, Villiers le Bel, France): sample volume, manager.UPLC coupled with quantification (s/n 10) = 80 ng L-1 in wastewater. 50 mL; cartridge, HLB Oasis cartridges a 5500QTRAP hybrid triple - Accuracy: relative recovery (200mg/6mL) from Waters. Washing with 5 mL quadrupole-linear ion trap mass spectrometer equipped (calculated with respect to of HPLC-water with a Turbo Ion Spray the IS, 100, 500, and 1,000 source from Applied ng L-1, n=5) = 47-73 %. 4º) After the elution with methanol, extracts Biosystems-Sciex (Foster - Repeatability: relative were evaporated to dryness City, California, USA). standard deviation (RSD, in - LC separation on a reversed- wastewater, spiking levels 5º) Reconstitution of the extract to 0.5mL of phase column Acquity HSS 100, 500, and -1 methanol–water (10:90, v/v). T3 column ( 50 mm×2,1 mm 1,000 ng L , n=5) = 10.9 %.
-1 i.d. 1,7 μm particle size; 6º) 5 μL of Internal standard (10 ng μL ) was Waters Corp. Milford, MA, added: Etoposide isotopically labelled USA) at flow rate of 0.4 mL min-1. The column was kept at 25 °. Ultrapure water 0.1 % formic acid (A) and acetonitrile (B), were employed as mobile phase (flow-rate 0.4 mL min-1). LC gradient : 0 min, 5 % of B: 0.1–4 min, 5-100% B; 4.0– 4.5 min, 100 % B; 4.5–5.0 min, 100–5 % B; 5.0 min, 5 % B during 0.5 min. Sample injection volume 5 μL - MS/MS conditions: positive ESI; SRM quantification transition, 589>185 collision energy, 75 eV; declustering potential, 56 V.
IF 1º) Filtration through 0.45 µm nylon membranes - Analysis by UPLC-MS/MS - Quantification by the Standard mixture was used for - Equipment: UPLC system isotope dilution method with calibration 2º) Addition of EDTA 0.1 M (Waters Corp.Milford, MA, calibration curves prepared USA) equipped with a binary in HPLC-water 3º) Concentration of water sample by SPE using solvent system (Milford, MA, - Linearity: 0.10–80 ng mL-1 the automatic extractor ASPECTM GX-271 USA) and a sample - Sensitivity: method limit of (Gilson, Villiers le Bel, France): sample volume, manager.UPLC coupled with quantification (s/n 10) = 5.8 ng L-1 in wastewater. 50 mL; cartridge, HLB Oasis cartridges a 5500QTRAP hybrid triple - Accuracy: relative recovery (200mg/6mL) from Waters. Washing with 5 mL quadrupole-linear ion trap mass spectrometer equipped (calculated with respect to of HPLC-water with a Turbo Ion Spray the IS, 100, 500, and 1,000 source from Applied ng L-1, n=5) = 74-90 %. 4º) After the elution with methanol, extracts Biosystems-Sciex (Foster - Repeatability: relative were evaporated to dryness City, California, USA). standard deviation (RSD, in - LC separation on a reversed- wastewater, spiking levels 5º) Reconstitution of the extract to 0.5mL of phase column Acquity HSS 100, 500, and -1 methanol–water (10:90, v/v). T3 column ( 50 mm×2,1 mm 1,000 ng L , n=5) = 3.7 %.
-1 i.d. 1,7 μm particle size; 6º) 5 μL of Internal standard (10 ng μL ) was Waters Corp. Milford, MA, added: Ifosfamide isotopically labelled USA) at flow rate of 0.4 mL min-1. The column was kept at 25 °. Ultrapure water 0.1 % formic acid (A) and acetonitrile (B), were employed as mobile phase (flow-rate 0.4 mL min-1). LC gradient : 0 min, 5 % of B: 0.1–4 min, 5-100% B; 4.0– 4.5 min, 100 % B; 4.5–5.0 min, 100–5 % B; 5.0 min, 5 % B during 0.5 min. Sample injection volume 5 μL - MS/MS conditions: positive ESI; SRM quantification transition, 261>154; SRM confirmation transition, 261 > 92; collision energy, 31 eV and 33 eV, respectively; declustering potential, 76 V.
MTX 1º) Filtration through 0.45 µm nylon membranes - Analysis by UPLC-MS/MS - Quantification by the Standard mixture was used for - Equipment: UPLC system isotope dilution method with calibration 2º) Addition of EDTA 0.1 M (Waters Corp.Milford, MA, calibration curves prepared USA) equipped with a binary in HPLC-water 3º) Concentration of water sample by SPE using solvent system (Milford, MA, - Linearity: 0.10–80 ng mL-1 the automatic extractor ASPECTM GX-271 USA) and a sample - Sensitivity: method limit of (Gilson, Villiers le Bel, France): sample volume, manager.UPLC coupled with quantification (s/n 10) = 5.9 ng L-1 in wastewater. 50 mL; cartridge, HLB Oasis cartridges a 5500QTRAP hybrid triple - Accuracy: relative recovery (200mg/6mL) from Waters. Washing with 5 mL quadrupole-linear ion trap mass spectrometer equipped (calculated with respect to of HPLC-water with a Turbo Ion Spray the IS, 100, 500, and 1,000 source from Applied ng L-1, n=5) = 82-87 %. 4º) After the elution with methanol, extracts Biosystems-Sciex (Foster - Repeatability: relative were evaporated to dryness City, California, USA). standard deviation (RSD, in - LC separation on a reversed- wastewater, spiking levels 5º) Reconstitution of the extract to 0.5mL of phase column Acquity HSS 100, 500, and -1 methanol–water (10:90, v/v). T3 column ( 50 mm×2,1 mm 1,000 ng L , n=5) = 3.0 %.
-1 i.d. 1,7 μm particle size; 6º) 5 μL of Internal standard (10 ng μL ) was Waters Corp. Milford, MA, added: Methotrexate isotopically labelled USA) at flow rate of 0.4 mL min-1. The column was kept at 25 °. Ultrapure water 0.1 % formic acid (A) and acetonitrile (B), were employed as mobile phase (flow-rate 0.4 mL min-1). LC gradient : 0 min, 5 % of B: 0.1–4 min, 5-100% B; 4.0– 4.5 min, 100 % B; 4.5–5.0 min, 100–5 % B; 5.0 min, 5 % B during 0.5 min. Sample injection volume 5 μL - MS/MS conditions: positive ESI; SRM quantification transition, 455>308; SRM confirmation transition, 455>175 ; collision energy, 27 eV and 57 eV, respectively; declustering potential, 131 V.