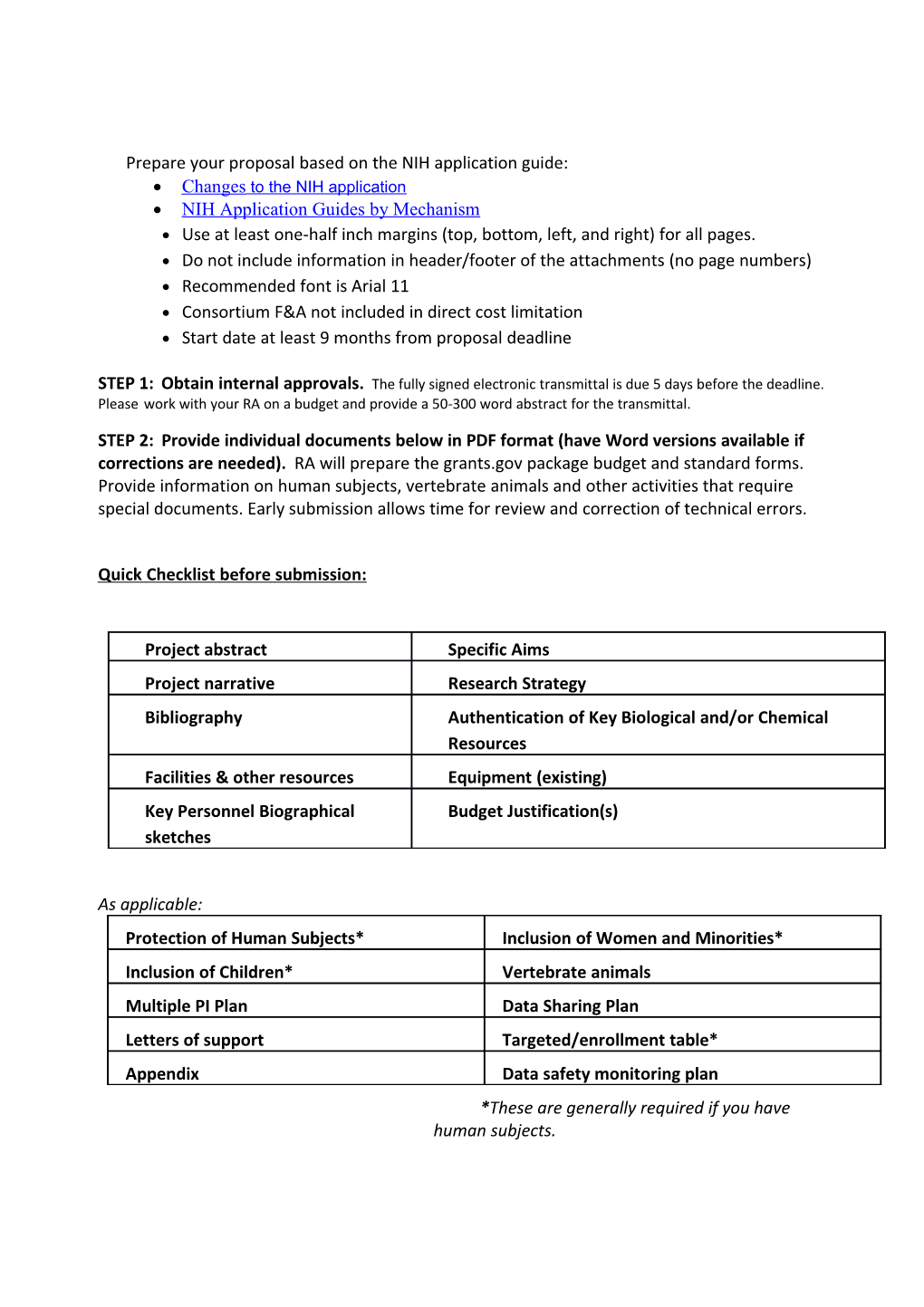
Prepare your proposal based on the NIH application guide: Changes to the NIH application NIH Application Guides by Mechanism Use at least one‐half inch margins (top, bottom, left, and right) for all pages. Do not include information in header/footer of the attachments (no page numbers) Recommended font is Arial 11 Consortium F&A not included in direct cost limitation Start date at least 9 months from proposal deadline
STEP 1: Obtain internal approvals. The fully signed electronic transmittal is due 5 days before the deadline. Please work with your RA on a budget and provide a 50‐300 word abstract for the transmittal.
STEP 2: Provide individual documents below in PDF format (have Word versions available if corrections are needed). RA will prepare the grants.gov package budget and standard forms. Provide information on human subjects, vertebrate animals and other activities that require special documents. Early submission allows time for review and correction of technical errors.
Quick Checklist before submission:
Project abstract Specific Aims Project narrative Research Strategy Bibliography Authentication of Key Biological and/or Chemical Resources Facilities & other resources Equipment (existing) Key Personnel Biographical Budget Justification(s) sketches
As applicable: Protection of Human Subjects* Inclusion of Women and Minorities* Inclusion of Children* Vertebrate animals Multiple PI Plan Data Sharing Plan Letters of support Targeted/enrollment table* Appendix Data safety monitoring plan *These are generally required if you have human subjects. Detailed Summary of Requirements
1. Cover letter –address genomic data if applicable
2. Assignment request form –let your RA know which institute(s) are preferred and reviewer information
3. Project abstract. 30 lines of text maximum.
4. Project narrative. A few sentences. Public health relevance statement
5. References/Bibliography
4. Facilities & Other Resources. Environment/institution investment contribution to success.
5. Equipment. Existing equipment used for the project. 6. Biographical sketches. 5 page maximum. Biosketch instructions
7. Budget Justification. Detailed budget justification unless modular budget (budgets under $250,000 direct costs/year, unless otherwise specified in guidelines).
For modular budgets, include the following as applicable: Modular Personnel Budget Justification: No dollar amounts, only effort of key personnel Other budget justification: Required only if requesting equipment or modules are not equal in each year Consortium justification: Budget justification for subcontracts
8. Specific Aims. 1 page maximum.
9. Research Strategy. Page limits by grant type
If you have human subjects (each one is a separate document) 10. Protection of Human Subjects 11. Inclusion of Women and Minorities 12. Inclusion of Children 13. Targeted/planned enrollment table
14. Vertebrate Animals: If you have vertebrate animals: http://grants.nih.gov/grants/olaw/vertebrate_animal_section.htm Confirm to your RA if the animals are euthanized. If yes, is method consistent with AVMA guidelines?
15. Multiple PI Plan: If you have multiple PIs
16. Data Sharing Plan: For applications requesting $500,000 or more in direct costs or as specified in the guidelines 9. Authentication of Key Biological and/or Chemical Resources: Briefly describe methods to ensure the identity and validity of key biological and/or chemical resources used in the proposed studies. 1 page 10. Appendix materials: These are restricted to surveys, consent forms, clinical trial protocols