Genetic Diversity and Host Range of Powdery Mildews on Papaveraceae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Overview of the Genus Phyllactinia (Ascomycota, Erysiphales) in Azerbaijan
Plant & Fungal Research (2018) 1(1): 9-17 © The Institute of Botany, ANAS, Baku, Azerbaijan http://dx.doi.org/10.29228/plantfungalres.2 December 2018 Overview of the genus Phyllactinia (Ascomycota, Erysiphales) in Azerbaijan Dilzara N. Aghayeva1 Key Words: distribution, ectoparasitism, endoparasit- Lamiya V. Abasova ism, host plant, plant pathogen, powdery mildews Institute of Botany, Azerbaijan National Academy of Sciences, Badamdar 40, Baku, AZ1004, Azerbaijan INTRODUCTION Susumu Takamatsu Graduate School of Bioresources, Mie University, Powdery mildews are one of the frequently encoun- 1577 Kurima-Machiya, Tsu 514-8507, Japan tered plant pathogens and most of them are epiphytic (14 genera from 18), in which they tend to produce hy- Abstract: Intergeneric diversity of powdery mildews phae and reproductive structures on surface of leaves, within the genus Phyllactinia in Azerbaijan was inves- young shoots and inflorescence [Braun, Cook, 2012; tigated using morphological approaches based on re-ex- Glawe, 2008]. These fungi absorb nutrients from plant amination of herbarium specimens kept in Mycological tissue via haustoria, which develops in epidermal cells Herbarium of the Institute of Botany (BAK), Azerbaijan of plants [Braun, Cook, 2012]. Among all powdery mil- National Academy of Sciences and collections of re- dews only four genera demonstrate endoparasitism, of cent years. To contribute detail taxonomic analysis data them Phyllactinia Lév., have partly endoparasitic na- given in literatures was revised. Modern taxonomic and ture. Fungi belonging to these genera penetrate into the nomenclature changes were considered. The host range plant cell via stomata and develop haustoria in paren- and geographical distribution of species residing to the chyma cells. Endoparasitic genera of powdery mildews genus within the country were clarified. -

New Powdery Mildew on Tomatoes
NEW POWDERY MILDEW ON TOMATOES Heather Scheck, Plant Pathologist Ag Commissioner’s Office, Santa Barbara County POWDERY MILDEW BIOLOGY Powdery mildew fungi are obligate, biotrophic parasites of the phylum Ascomycota of the Kingdom Fungi. The diseases they cause are common, widespread, and easily recognizable Individual species of powdery mildew fungi typically have a narrow host range, but the ones that infect Tomato are exceptionally large. Photo from APS Net POWDERY MILDEW BIOLOGY Unlike most fungal pathogens, powdery mildew fungi tend to grow superficially, or epiphytically, on plant surfaces. During the growing season, hyphae and spores are produced in large colonies that can coalesce Infections can also occur on stems, flowers, or fruit (but not tomato fruit) Our climate allows easy overwintering of inoculum and perfect summer temperatures for epidemics POWDERY MILDEW BIOLOGY Specialized absorption cells, termed haustoria, extend into the plant epidermal cells to obtain nutrition. Powdery mildew fungi can completely cover the exterior of the plant surfaces (leaves, stems, fruit) POWDERY MILDEW BIOLOGY Conidia (asexual spores) are also produced on plant surfaces during the growing season. The conidia develop either singly or in chains on specialized hyphae called conidiophores. Conidiophores arise from the epiphytic hyphae. This is the Anamorph. Courtesy J. Schlesselman POWDERY MILDEW BIOLOGY Some powdery mildew fungi produce sexual spores, known as ascospores, in a sac-like ascus, enclosed in a fruiting body called a chasmothecium (old name cleistothecium). This is the Teleomorph Chasmothecia are generally spherical with no natural opening; asci with ascospores are released when a crack develops in the wall of the fruiting body. -

Phylogeography of a Tertiary Relict Plant, Meconopsis Cambrica (Papaveraceae), Implies the Existence of Northern Refugia for a Temperate Herb
Article (refereed) - postprint Valtueña, Francisco J.; Preston, Chris D.; Kadereit, Joachim W. 2012 Phylogeography of a Tertiary relict plant, Meconopsis cambrica (Papaveraceae), implies the existence of northern refugia for a temperate herb. Molecular Ecology, 21 (6). 1423-1437. 10.1111/j.1365- 294X.2012.05473.x Copyright © 2012 Blackwell Publishing Ltd. This version available http://nora.nerc.ac.uk/17105/ NERC has developed NORA to enable users to access research outputs wholly or partially funded by NERC. Copyright and other rights for material on this site are retained by the rights owners. Users should read the terms and conditions of use of this material at http://nora.nerc.ac.uk/policies.html#access This document is the author’s final manuscript version of the journal article, incorporating any revisions agreed during the peer review process. Some differences between this and the publisher’s version remain. You are advised to consult the publisher’s version if you wish to cite from this article. The definitive version is available at http://onlinelibrary.wiley.com Contact CEH NORA team at [email protected] The NERC and CEH trademarks and logos (‘the Trademarks’) are registered trademarks of NERC in the UK and other countries, and may not be used without the prior written consent of the Trademark owner. 1 Phylogeography of a Tertiary relict plant, Meconopsis cambrica 2 (Papaveraceae), implies the existence of northern refugia for a 3 temperate herb 4 Francisco J. Valtueña*†, Chris D. Preston‡ and Joachim W. Kadereit† 5 *Área de Botánica, Facultad deCiencias, Universidad de Extremadura, Avda. de Elvas, s.n. -

Plant Science 2018: Resistance to Powdery Mildew (Blumeria Graminis F. Sp. Hordei) in Winter Barley, Poland- Jerzy H Czembor, Al
Extended Abstract Insights in Aquaculture and Biotechnology 2019 Vol.3 No.1 a Plant Science 2018: Resistance to powdery mildew (Blumeria graminis f. sp. hordei) in winter barley, Poland- Jerzy H Czembor, Aleksandra Pietrusinska and Kinga Smolinska-Plant Breeding and Acclimatization Institute – National Research Institute Jerzy H Czembor, Aleksandra Pietrusinska and Kinga Smolinska Plant Breeding and Acclimatization Institute – National Research Institute, Poland Powdery mildew (Blumeria graminis f. sp. hordei) is Barley powdery mildew is brought about by Blumeria the most ecomically important barley pathogen. This graminis f. sp. hordei (Bgh) is one of the most wind borne fungus causes foliar disease and yield damaging foliar maladies of grain. This growth is the loses rich up to 20-30%. Resistance for powdery main types of the family Blumeria however it has mildew is the aim of numerous breeding programmes. recently been treated as a types of Erysiphe. As per The transfer of the MLO gene for resistance to Braun (1987), it varies from all types of Erysiphe since powdery mildew into winter barley cultivars using its anamorph has special highlights, for instance, Marker-Assisted Selection (MAS) strategy is digitate haustoria, auxiliary mycelium with bristle-like presented. These cultivars are characterized by high hyphae and bulbous swellings of the conidiophores, and stable yield under polish conditions. Field testing and as a result of the structure of the ascocarps. Braun of the obtained lines with MLO resistance for their (1987) thinks about that, in view of these distinctions, agricultural value was conducted. Four cultivars there ought to be a detachment at conventional level. -

Leveillula Lactucae-Serriolaeon Lactucaserriola in Jordan
Phytopathologia Mediterranea Firenze University Press The international journal of the www.fupress.com/pm Mediterranean Phytopathological Union New or Unusual Disease Reports Leveillula lactucae-serriolae on Lactuca serriola in Jordan Citation: Lebeda A., Kitner M., Mieslerová B., Křístková E., Pavlíček T. (2019) Leveillula lactucae-serriolae on Lactuca serriola in Jordan. Phytopatho- Aleš LEBEDA1,*, Miloslav KITNER1, Barbora MIESLEROVÁ1, Eva logia Mediterranea 58(2): 359-367. doi: KŘÍSTKOVÁ1, Tomáš PAVLÍČEK2 10.14601/Phytopathol_Mediter-10622 1 Department of Botany, Faculty of Science, Palacký University in Olomouc, Šlechtitelů Accepted: February 7, 2019 27, Olomouc, CZ-78371, Czech Republic 2 Institute of Evolution, University of Haifa, Mount Carmel, Haifa 31905, Israel Published: September 14, 2019 Corresponding author: [email protected] Copyright: © 2019 Lebeda A., Kit- ner M., Mieslerová B., Křístková E., Pavlíček T.. This is an open access, Summary. Jordan contributes significantly to the Near East plant biodiversity with peer-reviewed article published by numerous primitive forms and species of crops and their wild relatives. Prickly lettuce Firenze University Press (http://www. (Lactuca serriola) is a common species in Jordan, where it grows in various habitats. fupress.com/pm) and distributed During a survey of wild Lactuca distribution in Jordan in August 2007, plants of L. under the terms of the Creative Com- serriola with natural infections of powdery mildew were observed at a site near Sho- mons Attribution License, which per- bak (Ma’an Governorate). Lactuca serriola leaf samples with powdery mildew infec- mits unrestricted use, distribution, and tions were collected from two plants and the pathogen was analyzed morphologically. reproduction in any medium, provided Characteristics of the asexual and sexual forms were obtained. -

Basal Resistance of Barley to Adapted and Non-Adapted Forms of Blumeria Graminis
Basal resistance of barley to adapted and non-adapted forms of Blumeria graminis Reza Aghnoum Thesis committee Thesis supervisors Prof. Dr. Richard G.F. Visser Professor of Plant Breeding Wageningen University Dr.ir. Rients E. Niks Assistant professor, Laboratory of Plant Breeding Wageningen University Other members Prof. Dr. R.F. Hoekstra, Wageningen University Prof. Dr. F. Govers, Wageningen University Prof. Dr. ir. C. Pieterse, Utrecht University Dr.ir. G.H.J. Kema, Plant Research International, Wageningen This research was conducted under the auspices of the Graduate school of Experimental Plant Sciences. II Basal resistance of barley to adapted and non-adapted forms of Blumeria graminis Reza Aghnoum Thesis Submitted in partial fulfillment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. Dr. M.J. Kropff, in the presence of the Thesis Committee appointed by the Doctorate Board to be defended in public on Tuesday 16 June 2009 at 4 PM in the Aula. III Reza Aghnoum Basal resistance of barley to adapted and non-adapted forms of Blumeria graminis 132 pages. Thesis, Wageningen University, Wageningen, NL (2009) With references, with summaries in Dutch and English ISBN 978-90-8585-419-7 IV Contents Chapter 1 1 General introduction Chapter 2 15 Which candidate genes are responsible for natural variation in basal resistance of barley to barley powdery mildew? Chapter 3 47 Transgressive segregation for extreme low and high level of basal resistance to powdery mildew in barley -

Studies in <I>Erysiphales</I> Anamorphs (4): Species on <I>Hydrangeaceae</I> and <I>Papaveraceae&L
ISSN (print) 0093-4666 © 2011. Mycotaxon, Ltd. ISSN (online) 2154-8889 MYCOTAXON Volume 115, pp. 287–301 January–March 2011 doi: 10.5248/115.287 Studies in Erysiphales anamorphs (4): species on Hydrangeaceae and Papaveraceae Anke Schmidt1 & Markus Scholler2* 1Holunderweg 2 B, D-23568 Lübeck, Germany 2Staatliches Museum für Naturkunde, Erbprinzenstr. 13, D-76133 Karlsruhe, Germany * Correspondence to: [email protected] Abstract — Anamorphic powdery mildews on Hydrangeaceae and Papaveraceae in Germany are revised. Species are documented in detail including line drawings, photomicrographs, and identification keys. On Papaveraceae three species are accepted, specifically Erysiphe macleayae on Chelidonium majus and Macleaya cordata, E. cruciferarum on Eschscholzia californica, and Oidium sp. (an unknown species previously assigned to E. cruciferarum) on Pseudofumaria lutea. Species on Hydrangeaceae are Oidium hortensiae on Hydrangea macrophylla and E. deutziae on Deutzia cf. scabra and Philadelphus cf. coronarius. The fungus on the latter host plant was previously assigned to O. hortensiae. Erysiphe deutziae, E. macleayae, and Oidium hortensiae are introduced species. Key words — conidial germination, morphology, neomycete Introduction In Germany, there are three species of Erysiphales reported on Papaveraceae (Erysiphe cruciferarum, Erysiphe cf. macleayae, Golovinomyces orontii (Castagne) Heluta); and two (Erysiphe deutziae, Oidium hortensiae) on Hydrangeaceae (Braun 1995, Jage et. al. 2010). The following is a revision of anamorphs on certain host plants of Papaveraceae/Hydrangeaceae (Chelidonium, Deutzia, Hydrangea, Macleaya, Meconopsis, Philadelphus and Pseudofumaria) for which the host/pathogen affiliations have been doubtful. Materials & methods Both fresh and dried structures were examined in tap water mounts with light microscopy using Olympus BH 2 and Zeiss Axioskop 2 Plus. -

Preliminary Classification of Leotiomycetes
Mycosphere 10(1): 310–489 (2019) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/10/1/7 Preliminary classification of Leotiomycetes Ekanayaka AH1,2, Hyde KD1,2, Gentekaki E2,3, McKenzie EHC4, Zhao Q1,*, Bulgakov TS5, Camporesi E6,7 1Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China 2Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai, 57100, Thailand 3School of Science, Mae Fah Luang University, Chiang Rai, 57100, Thailand 4Landcare Research Manaaki Whenua, Private Bag 92170, Auckland, New Zealand 5Russian Research Institute of Floriculture and Subtropical Crops, 2/28 Yana Fabritsiusa Street, Sochi 354002, Krasnodar region, Russia 6A.M.B. Gruppo Micologico Forlivese “Antonio Cicognani”, Via Roma 18, Forlì, Italy. 7A.M.B. Circolo Micologico “Giovanni Carini”, C.P. 314 Brescia, Italy. Ekanayaka AH, Hyde KD, Gentekaki E, McKenzie EHC, Zhao Q, Bulgakov TS, Camporesi E 2019 – Preliminary classification of Leotiomycetes. Mycosphere 10(1), 310–489, Doi 10.5943/mycosphere/10/1/7 Abstract Leotiomycetes is regarded as the inoperculate class of discomycetes within the phylum Ascomycota. Taxa are mainly characterized by asci with a simple pore blueing in Melzer’s reagent, although some taxa have lost this character. The monophyly of this class has been verified in several recent molecular studies. However, circumscription of the orders, families and generic level delimitation are still unsettled. This paper provides a modified backbone tree for the class Leotiomycetes based on phylogenetic analysis of combined ITS, LSU, SSU, TEF, and RPB2 loci. In the phylogenetic analysis, Leotiomycetes separates into 19 clades, which can be recognized as orders and order-level clades. -

Anali Za Istrske in Mediteranske Študije Annali Di Studi Istriani E Mediterranei Annals for Istrian and Mediterranean Studies Series Historia Naturalis, 30, 2020, 2
Anali za istrske in mediteranske študije Annali di Studi istriani e mediterranei Annals for Istrian and Mediterranean Studies Series Historia Naturalis, 30, 2020, 2 UDK 5 Annales, Ser. hist. nat., 30, 2020, 2, pp. 131-290, Koper 2020 ISSN 1408-533X UDK 5 ISSN 1408-533X e-ISSN 2591-1783 Anali za istrske in mediteranske študije Annali di Studi istriani e mediterranei Annals for Istrian and Mediterranean Studies Series Historia Naturalis, 30, 2020, 2 KOPER 2020 ANNALES · Ser. hist. nat. · 30 · 2020 · 2 Anali za istrske in mediteranske študije - Annali di Studi istriani e mediterranei - Annals for Istrian and Mediterranean Studies ISSN 1408-533X UDK 5 Letnik 30, leto 2020, številka 2 e-ISSN 2591-1783 Alessandro Acquavita (IT), Nicola Bettoso (IT), Christian Capapé (FR), UREDNIŠKI ODBOR/ Darko Darovec, Dušan Devetak, Jakov Dulčić (HR), Serena Fonda COMITATO DI REDAZIONE/ Umani (IT), Andrej Gogala, Daniel Golani (IL), Danijel Ivajnšič, BOARD OF EDITORS: Mitja Kaligarič, Marcelo Kovačič (HR), Andrej Kranjc, Lovrenc Lipej, Vesna Mačić (ME), Alenka Malej, Patricija Mozetič, Martina Orlando- Bonaca, Michael Stachowitsch (AT), Tom Turk, Al Vrezec Glavni urednik/Redattore capo/ Editor in chief: Darko Darovec Odgovorni urednik naravoslovja/ Redattore responsabile per le scienze naturali/Natural Science Editor: Lovrenc Lipej Urednica/Redattrice/Editor: Martina Orlando-Bonaca Lektor/Supervisione/Language editor: Petra Berlot Kužner (angl.) Prevajalci/Traduttori/Translators: Martina Orlando-Bonaca (sl./it.) Oblikovalec/Progetto grafico/ Graphic design: -

Illustrated Flora of East Texas Illustrated Flora of East Texas
ILLUSTRATED FLORA OF EAST TEXAS ILLUSTRATED FLORA OF EAST TEXAS IS PUBLISHED WITH THE SUPPORT OF: MAJOR BENEFACTORS: DAVID GIBSON AND WILL CRENSHAW DISCOVERY FUND U.S. FISH AND WILDLIFE FOUNDATION (NATIONAL PARK SERVICE, USDA FOREST SERVICE) TEXAS PARKS AND WILDLIFE DEPARTMENT SCOTT AND STUART GENTLING BENEFACTORS: NEW DOROTHEA L. LEONHARDT FOUNDATION (ANDREA C. HARKINS) TEMPLE-INLAND FOUNDATION SUMMERLEE FOUNDATION AMON G. CARTER FOUNDATION ROBERT J. O’KENNON PEG & BEN KEITH DORA & GORDON SYLVESTER DAVID & SUE NIVENS NATIVE PLANT SOCIETY OF TEXAS DAVID & MARGARET BAMBERGER GORDON MAY & KAREN WILLIAMSON JACOB & TERESE HERSHEY FOUNDATION INSTITUTIONAL SUPPORT: AUSTIN COLLEGE BOTANICAL RESEARCH INSTITUTE OF TEXAS SID RICHARDSON CAREER DEVELOPMENT FUND OF AUSTIN COLLEGE II OTHER CONTRIBUTORS: ALLDREDGE, LINDA & JACK HOLLEMAN, W.B. PETRUS, ELAINE J. BATTERBAE, SUSAN ROBERTS HOLT, JEAN & DUNCAN PRITCHETT, MARY H. BECK, NELL HUBER, MARY MAUD PRICE, DIANE BECKELMAN, SARA HUDSON, JIM & YONIE PRUESS, WARREN W. BENDER, LYNNE HULTMARK, GORDON & SARAH ROACH, ELIZABETH M. & ALLEN BIBB, NATHAN & BETTIE HUSTON, MELIA ROEBUCK, RICK & VICKI BOSWORTH, TONY JACOBS, BONNIE & LOUIS ROGNLIE, GLORIA & ERIC BOTTONE, LAURA BURKS JAMES, ROI & DEANNA ROUSH, LUCY BROWN, LARRY E. JEFFORDS, RUSSELL M. ROWE, BRIAN BRUSER, III, MR. & MRS. HENRY JOHN, SUE & PHIL ROZELL, JIMMY BURT, HELEN W. JONES, MARY LOU SANDLIN, MIKE CAMPBELL, KATHERINE & CHARLES KAHLE, GAIL SANDLIN, MR. & MRS. WILLIAM CARR, WILLIAM R. KARGES, JOANN SATTERWHITE, BEN CLARY, KAREN KEITH, ELIZABETH & ERIC SCHOENFELD, CARL COCHRAN, JOYCE LANEY, ELEANOR W. SCHULTZE, BETTY DAHLBERG, WALTER G. LAUGHLIN, DR. JAMES E. SCHULZE, PETER & HELEN DALLAS CHAPTER-NPSOT LECHE, BEVERLY SENNHAUSER, KELLY S. DAMEWOOD, LOGAN & ELEANOR LEWIS, PATRICIA SERLING, STEVEN DAMUTH, STEVEN LIGGIO, JOE SHANNON, LEILA HOUSEMAN DAVIS, ELLEN D. -
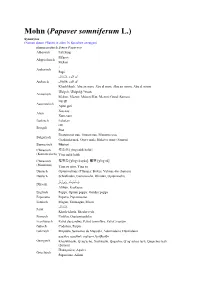
Mohn (Papaver Somniferum
Mohn (Papaver somniferum L.) Synonyme (Namen dieser Pflanze in allen 76 Sprachen anzeigen) pharmazeutisch Semen Papaveris Albanisch Lulëkuqe Μήκων Altgriechisch Mekon ፓፓ Amharisch Papi , Arabisch , Khashkhash, Abu an-num, Abu al-num, Abu an-noom, Abu al-noom Մեկոն, Մեկոնի Կուտ Armenisch Mekon, Megon; Mekoni Kut, Megoni Good (Samen) Assamesisch Xaş-xaş Azeri Хаш-хаш Baskisch Lobelarr Bengali Градински мак, Опиев мак, Маково семе Bulgarisch Gradinski mak, Opiev mak; Makovo seme (Samen) Burmesisch Chinesisch 櫻粟殼 [yìng suhk hohk] (Kantonesisch) Ying suhk hohk Chinesisch 櫻粟殼 [yīng sù qiào], 罂粟 [yīng sù] (Mandarin) Ying su qiao, Ying su Dänisch Opiumvalmue (Pflanze); Birkes, Valmue-frø (Samen) Deutsch Schlafmohn, Gartenmohn, Ölmohn, Opiummohn, , Dhivehi Afihun, Kaskasaa Englisch Poppy, Opium poppy, Garden poppy Esperanto Papavo, Papavosemo Estnisch Magun, Unimagun, Moon Farsi Khash-khash, Shagheyegh Finnisch Unikko, Oopiumiunikko Französisch Pavot des jardins, Pavot somnifère, Pavot à opium Gälisch Codalion, Paipin Galizisch Mapoula, Sementes de Mapoula, Adormideira, Durmideira ყაყაჩო, ყაყაჩოს თესლი, ხოშხოში Georgisch Khoshkhoshi, Q’aq’acho, Xoshxoshi, Qaqacho; Q’aq’achos tesli, Qaqachos tesli (Samen) Παπαρούνα, Αθιόνι Griechisch Paparouna, Afioni Gujarati Hebräisch Pereg Hindi Irisch Poipín Isländisch Valmúafræ, Birki Italienisch Papavero, Papavero sonnifero 芥子, 罌粟 けし Japanisch ケシ, ポピー Keshi, Papi , Jiddisch Mondl, Mon Kannada Көкнәр Kasachisch Köknär Kashmiri Khash-khash Katalanisch Cascall, Herba dormidora 아편, 포피, 양귀비 Koreanisch Apyeon, Apyon, -

Role of MLO Genes in Susceptibility to Powdery Mildew in Apple and Grapevine
Role of MLO genes in susceptibility to powdery mildew in apple and grapevine Stefano Pessina 1 Thesis committee Promotor Prof. Dr. Richard G. F. Visser Professor of Plant Breeding, Wageningen UR Co-promotors Dr. Henk J. Schouten Senior Scientist, Wageningen UR Plant Breeding Dr. Mickael Malnoy Senior Scientist, Fondazione Edmund Mach, San Michele all’Adige (Italy) Dr. Yuling Bai, Associate professor Wageningen UR Plant Breeding Opponents Prof. Dr. Bart P. H. J. Thomma (Wageningen University) Prof. Dr. Ralph Panstruga (Aachen University) Dr. Bert J. Meulenbroek (Fresh Forward) Dr. Theo A. J. van der Lee (Wageningen University) This research was conducted under the auspices of the Graduate School in Experimental Plant Sciences 2 Role of MLO genes in susceptibility to powdery mildew in apple and grapevine Stefano Pessina Thesis submitted in fulfilment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. Dr. Arthur Mol In the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Friday 22 January 2016 at 1.30 p.m. in the Aula 3 Stefano Pessina Role of MLO genes in susceptibility to powdery mildew in apple and grapevine 222 pages Phd thesis, Wageningen University, Wageningen NL (2016) With references, with summary in English. ISBN 978-94-6257-620-9 4 Table of contents CHAPTER 1 General Introduction 7 CHAPTER 2 Characterization of the MLO gene family in 29 Rosaceae and gene expression analysis in Malus domestica CHAPTER 3 Knock-down of the