Diagnostic Virology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
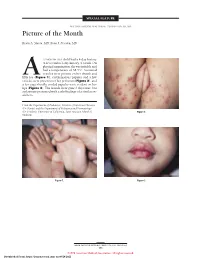
Picture of the Month
SPECIAL FEATURE SECTION EDITOR: WALTER W. TUNNESSEN, JR, MD Picture of the Month Kevin A. Slavin, MD; Ilona J. Frieden, MD 15-MONTH-OLD child had a 4-day history of fever and a 1-day history of a rash. On physical examination she was irritable and had a temperature of 38.3°C. Scattered vesicles were present on her thumb and Afifth toe (Figure 1), erythematous papules and a few vesicles were present over her perineum (Figure 2), and a few superficially eroded papules were evident on her lips (Figure 3). The lesions were gone 3 days later, but a playmate presented with early findings of a similar ex- anthem. From the Department of Pediatrics, Division of Infectious Diseases (Dr Slavin) and the Department of Pediatrics and Dermatology (Dr Frieden), University of California, San Francisco School of Figure 2. Medicine. Figure 1. Figure 3. ARCH PEDIATR ADOLESC MED/ VOL 152, MAY 1998 505 ©1998 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 09/28/2021 Denouement and Discussion Hand-Foot-and-Mouth Disease Figure 1. Vesicles are present on the thumb and fifth toe. foot-and-mouth disease).1,2,5-9 The lesions on the but- tocks are of the same size and typical of the early forms Figure 2. Multiple erythematous papules and a few scattered vesicles are of the exanthem, but they are not frequently vesicular present over the perineum. in nature. Lesions involving the perineum seem to be more Figure 3. Superficially eroded papules are present on the lips. common in children who wear diapers, suggesting that friction or minor trauma may play a role in the develop- ment of lesions. -

Hand, Foot, and Mouth Disease (Coxsackievirus) Fact Sheet
Hand, Foot, and Mouth Disease (Coxsackievirus) Fact Sheet Hand, foot, and mouth disease is caused by one of several types of viruses Hand, foot, and mouth disease is usually characterized by tiny blisters on the inside of the mouth and the palms of the hands, fingers, soles of the feet. It is commonly caused by coxsackievirus A16 (an enterovirus), and less often by other types of viruses. Anyone can get hand, foot, and mouth disease Young children are primarily affected, but it may be seen in adults. Most cases occur in the summer and early fall. Outbreaks may occur among groups of children especially in child care centers or nursery schools. Symptoms usually appear 3 to 5 days after exposure. Hand, foot, and mouth disease is usually spread through person-to-person contact People can spread the disease when they are shedding the virus in their feces. It is also spread by the respiratory tract from mouth or respiratory secretions (such as from saliva on hands or toys). The virus has also been found in the fluid from the skin blisters. The infection is spread most easily during the acute phase/stage of illness when people are feeling ill, but the virus can be spread for several weeks after the onset of infection. The symptoms are much like a common cold with a rash The rash appears as blisters or ulcers in the mouth, on the inner cheeks, gums, sides of the tongue, and as bumps or blisters on the hands, feet, and sometimes other parts of the skin. The skin rash may last for 7 to 10 days. -

Hand, Foot and Mouth Disease
CDAlert Monthly Newsletter of National Institute of Communicable Diseases, Directorate General of Health Services, Government of India July 2008 Vol.12 : No.3 Hand, Foot and Mouth Disease INTRODUCTION Enterovirus71 or other enteroviruses have also been associated with HFMD and with outbreaks of the Hand, foot and mouth disease (HFMD) is a human disease. HFMD caused by Enterovirus71 has shown syndrome caused by intestinal viruses of the a higher incidence of neurologic involvement. Fatal Picornaviridae family. The most common strains cases of encephalitis caused by enterovirus 71 have causing HFMD are Coxsackie A virus and occurred during outbreaks. Enterovirus71 (EV71). It is a common viral illness of infants and children and is extremely uncommon To Summarise: in adults; however, still a possibility. Most adults Ø Coxsackie virus A16®Mild Infection HFMD have strong enough immune systems to defend the virus, but those with immune deficiencies are very Ø Enterovirus71®HFMD with severe neurologic susceptible. involvement including fatal encephalitis It is often confused with foot-and-mouth (also called Ø Other enteroviruses hoof-and-mouth) disease, a disease of cattle, sheep, RECORDED OUTBREAKS and swine; however, the two diseases are not Individual cases and outbreaks of HFMD occur related—they are caused by different viruses. worldwide. In temperate climates, cases occur more Humans do not get the animal disease, and animals often in summer and early autumn. Since 1997, do not get the human disease. outbreaks of HFMD caused by Enterovirus 71 have AETIOLOGY been reported in Asia and Australia. HFMD is caused by viruses belonging to the Ø In 1997, 34 children died in an outbreak in enterovirus genus (group). -

A Guide to Clinical Management and Public Health Response for Hand, Foot and Mouth Disease (HFMD)
A Guide to Clinical Management and Public Health Response for Hand, Foot and Mouth Disease (HFMD) WHO Western Pacific Region PUBLICATION ISBN-13 978 92 9061 525 5 A Guide to Clinical Management and Public Health Response for Hand, Foot and Mouth Disease (HFMD) WHO Library Cataloguing in Publication Data A Guide to clinical management and public health response for hand, foot and mouth disease (HFMD) 1. Hand, foot and mouth disease – epidemiology. 2. Hand, foot and mouth disease – prevention and control. [ ii ] 3. Disease outbreaks. 4. Enterovirus A, Human. I. Regional Emerging Disease Intervention Center. ISBN 978 92 9061 525 5 (NLM Classification: WC 500) © World Health Organization 2011 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). For WHO Western Pacific Regional Publications, request for permission to reproduce should be addressed to the Publications Office, World Health Organization, Regional Office for the Western Pacific, P.O. Box 2932, 1000, Manila, Philippines, (fax: +632 521 1036, e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Diseases in Search of Viruses
DISEASES IN SEARCH OF VIRUSES BY C. H. STUART-HARRIS, M.D., F.R.C.P. Professor of Medicine, University of Sheffield* ft f>]/ery?ne agrees that tremendous changes have occurred in the past few years in the ?f the infectious diseases. Formidable have been both for the tt? weapons forged a c atlftent and the prevention of bacterial infections. As result, diphtheria has be- CQ^e almost extinct and the enteric diseases are much reduced, patients with strepto- 1) ^ disease and with pneumonia less often require hospital treatment, and the acute i^ness *n childhood has Virus infections have 1?t K considerably lightened. W Wever> altered in the same way or to the same extent. Some, such as poliomyelitis, a to both children and adults. undescribed S(J ? become threat Previously conditions as myalgic encephalomyelitis or Royal Free disease, aseptic meningitis with an and little known syndromes such as herpangina, pharyngo-conjunctival fever a^hemOrnholm disease have achieved a of interest and even of as cjjj position importance of ,S.es of morbidity. Moreover, the development of new techniques for the cultivation has led to the realization of a method of control of certain diseases and to a H^lr.Usesk 'cia regard to diagnosis. These changes have affected the work of both clin- oUtlns and microbiologists, and yet there has also arisen a considerable divergence of iti h?0^ between these two groups of medical men. One could almost say that they live -j,,0 different worlds. virune virologist, being equipped with new techniques for the laboratory study of CoVeSes> has been more and more excited over the possibility of fundamental dis- w concerning the nature of viruses, of their mode of multiplication and of the Uity restraining their multiplication by chemical substances. -

The Medical and Public Health Importance of the Coxsackie Viruses
806 S.A. MEDICAL JOURNAL 25 August 1956 it seems that this mother's claim cannot be upheld, Dit lyk dus of hierdie moeder se eis nie gestaaf kan word and we must still look for further evidence of human nie en ons moet nog steeds soek na verdere bewyse van parthenogenesis. menslike partenogenese. 1. Editorial (1955): Lancet, 2, 967. 1. Van die Redaksie (1955): Lancet, 2, 967. 2. Pincus, G. and Shapiro, H. (1939): Proc. Nat. Acad. Sci., 2. Pincus, G. en Shapiro, H. (1939): Proc. Nat. Acad. Sci., 26,163. 26, 163. 3. Balfour-Lynn, S. (1956): Lancet, 1, 1072. 3. Balfour-Lynn, S. (1956): Lancet, 1, 1072. THE MEDICAL AND PUBLIC HEALTH IMPORTANCE OF THE COXSACKIE VIRUSES JA.\1ES GEAR, V.MasRoCH M'D F. R. PRINSLOO Poliomyelitis Research Foundation, South African Institute for Medical Research The Coxsackie group of viruses derived their name paralytic poliomyelitis infected also with poliovirus. from the Hudson River Town, Coxsackie, in New York As a result of more recent studies their pathogenicity State, where the first two members of this group were has now been more clearly defined. 2 The group-A identified by Dalldorf and Sickies in 1947.1 Both these viruses have been incriminated as the cause of herp viruses were isolated in suckling mice from the faeces angina and, as the present studies show, are possibly of children acutely ill with paralytic poliomyelitis. The related to a number of other illnesses. Coxsackie-B pathogenicity for suckling mice is one of the distinguish viruses have been incriminated as the cause of Bornholm ing features of this group of viruses, and their relative disease and, as the present studies show, in parts of lack of pathogenicity for adult mice and other experi Southern Africa are important causes ofaseptic meningo mental animals accounts for their escape from recogni encephalitis and of myocarditis neonatorum. -

82171915.Pdf
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector International Journal of Infectious Diseases 49 (2016) 80–86 Contents lists available at ScienceDirect International Journal of Infectious Diseases jou rnal homepage: www.elsevier.com/locate/ijid Simultaneous detection of 13 viruses involved in meningoencephalitis using a newly developed multiplex PCR Mag-array system a,1 a,1 a a a a a Xiaodan Shi , Rui Wu , Ming Shi , Linfu Zhou , Mengli Wu , Yining Yang , Xinyue An , a a b b, a, Wen Dai , Liang Tian , Chen Zhang , Xuejun Ma **, Gang Zhao * a Department of Neurology, Xijing Hospital, The Fourth Military Medical University, Changle-Xi Road 167, Xi’an 710032, China b Key Laboratory for Medical Virology, Ministry of Health, National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, No.155, Changbai Road, Changping District, Beijing 100050, China A R T I C L E I N F O S U M M A R Y Article history: Background: The early detection and identification of pathogens in central nervous system viral Received 14 December 2015 infections associated with neurological disease increases the survival rate. However, the limitations of Received in revised form 27 April 2016 current diagnostic methods contribute to a lack of proper diagnosis in 62% of patients. Therefore, a robust Accepted 19 May 2016 method for detecting multiple viruses in a single reaction with high specificity, throughput, and speed is required. Keywords: Methods: A multiplex PCR Mag-Array (MPMA) system was developed that integrates three strategies: MPMA system chimeric primer design, temperature switch PCR, and MagPlex-TAG techniques. -

Guidelines for Occupational Health Follow up of Communicable Diseases for Manager/Supervisors
Winnipeg Regional Health Authority Occupational and Environmental Safety & Health (OESH) Guidelines for Occupational Health Follow Up of Communicable Diseases For Manager/Supervisors Page | 1 2019.02.04 version 2 Winnipeg Regional Health Authority Occupational and Environmental Safety & Health (OESH) INTRODUCTION ................................................................................................................................................................................. 3 WORKERS COMPENSATION BOARD (WCB) CLAIMS ........................................................................................................................... 5 ANTIBIOTIC RESISTANT ORGANISMS (AROS) ..................................................................................................................................... 6 BLOOD AND BODY FLUID EXPOSURES ............................................................................................................................................... 9 CIMEX LECTULARIUS (BED BUGS) .................................................................................................................................................... 10 CREUTZFELDT-JAKOB DISEASE (CJD) ................................................................................................................................................ 11 DIARRHEA (BACTERIAL, CLOSTRIDIUM DIFFICILE (C. DIFFICILE), VIRAL) ........................................................................................... 12 GROUP A STREPTOCOCCUS ............................................................................................................................................................ -

Hand-Foot-And-Mouth Disease Caused by Coxsackievirus A6 on the Rise
Hand-foot-and-mouth Disease Caused by Coxsackievirus A6 on the Rise Brooks David Kimmis, MD; Christopher Downing, MD; Stephen Tyring, MD, PhD CVA16 has traditionally been the primary strain caus- PRACTICE POINTS ing HFMD, CVA6 has become a major cause of HFMD • Coxsackievirus A6 is an increasingly more common outbreaks in the United States and worldwide in recent cause of hand-foot-and-mouth disease (HFMD), years.6-12 Interestingly, CVA6 also has been found to be often with atypical presentation, more severe disease, associated with adult HFMD, which has increased in and association with HFMD in adults. incidence. The CVA6 strain was first identified in associa- • Coxsackievirus A6 has become a major cause of tion with the disease during HFMD outbreaks in Finland HFMD outbreak in the United States and worldwide. and Singapore in 2008,13,14 with similar strains detected in subsequent outbreaks in Taiwan, Japan, Spain, France, China, India, and the United States.12,15-25 Most cases took place in warmer months, with one winter outbreak in Hand-foot-and-mouth disease (HFMD) is a viral illness caused Massachusetts in 2012.24 most commonly by coxsackievirus A16 (CVA16) and enterovirus 71 (EV71). The disease mainly affects pediatric populations younger Herein, we review the incidence of CVA6, as well as than 5 years and is characterized by lesions of the oral mucosa, its atypical presentation, diagnosis, and treatment to aid palms, and soles, lasting for 7 to 10 days. In recent years, CVA6 has dermatologists. Given the increasing incidence of HFMD become a major cause of HFMD outbreaks in the United States and caused by CVA6 and its often atypical presentation, it is worldwide. -

Coxsackievirus B
PRINCIPLE OF THE IFA PROCEDURE The MBL Bion COXSACKIEVIRUS B ANTIGEN SUBSTRATE SLIDES may be utilized in the indirect fluorescent antibody assay method first described by Weller COXSACKIEVIRUS B and Coons11 and further developed by Riggs, et al.12 The procedure is carried out in two basic reaction steps: ANTIGEN SUBSTRATE SLIDE Step 1 - Human serum is reacted with the antigen substrate. Antibodies, if present, will bind to the antigen forming stable antigen-antibody complexes. If no antibodies are present, the complexes will not be PRODUCT AVAILABILITY formed and serum components will be washed away. The following Coxsackievirus Group B Antigen Substrate Slides are available Step 2 - Fluorescein labeled antihuman IgG (or IgM) antibody is added to the individually from MBL Bion: reaction site which binds with the complexes formed in step one. This results in a positive reaction of bright apple-green fluorescence when Antigen Substrate Slide Code No. Code No. viewed with a properly equipped fluorescence microscope. If no complexes Coxsackievirus B (1-6) Screen CB-3312 CB-3306 are formed in step one, the fluorescein labeled antibody will be washed Number of Tests 12-Well 6-Well away, exhibiting a negative result. ( = Code Number) REAGENTS INTENDED USE MBL Bion COXSACKIEVIRUS B ANTIGEN SUBSTRATE SLIDES are The MBL Bion COXSACKIEVIRUS B ANTIGEN SUBSTRATE SLIDES may individually foil-wrapped slides of six or twelve wells with a mixture of be used as the antigenic substrate in indirect fluorescent antibody assays for Coxsackievirus B (1-6; NIH strains) infected and uninfected A549 cells fixed the qualitative and/or semi-quantitative determination of Coxsackievirus B onto each well. -

Clinical Syndromes/Conditions with Required Level Or Precautions
Clinical Syndromes/Conditions with Required Level or Precautions This resource is an excerpt from the Best Practices for Routine Practices and Additional Precautions (Appendix N) and was reformatted for ease of use. For more information please contact Public Health Ontario’s Infection Prevention and Control Department at [email protected] or visit www.publichealthontario.ca Clinical Syndromes/Conditions with Required Level or Precautions This is an excerpt from the Best Practices for Routine Practices and Additional Precautions (Appendix N) Table of Contents ABSCESS DECUBITUS ULCER HAEMORRHAGIC FEVERS NOROVIRUS SMALLPOX OPHTHALMIA ADENOVIRUS INFECTION DENGUE HEPATITIS, VIRAL STAPHYLOCOCCAL DISEASE NEONATORUM AIDS DERMATITIS HERPANGINA PARAINFLUENZA VIRUS STREPTOCOCCAL DISEASE AMOEBIASIS DIARRHEA HERPES SIMPLEX PARATYPHOID FEVER STRONGYLOIDIASIS ANTHRAX DIPHTHERIA HISTOPLASMOSIS PARVOVIRUS B19 SYPHILIS ANTIBIOTIC-RESISTANT EBOLA VIRUS HIV PEDICULOSIS TAPEWORM DISEASE ORGANISMS (AROs) ARTHROPOD-BORNE ECHINOCOCCOSIS HOOKWORM DISEASE PERTUSSIS TETANUS VIRAL INFECTIONS ASCARIASIS ECHOVIRUS DISEASE HUMAN HERPESVIRUS PINWORMS TINEA ASPERGILLOSIS EHRLICHIOSIS IMPETIGO PLAGUE TOXOPLASMOSIS INFECTIOUS BABESIOSIS ENCEPHALITIS PLEURODYNIA TOXIC SHOCK SYNDROME MONONUCLEOSIS ENTEROBACTERIACEAE- BLASTOMYCOSIS INFLUENZA PNEUMONIA TRENCHMOUTH RESISTANT BOTULISM ENTEROBIASIS KAWASAKI SYNDROME POLIOMYELITIS TRICHINOSIS PSEUDOMEMBRANOUS BRONCHITIS ENTEROCOLITIS LASSA FEVER TRICHOMONIASIS COLITIS -

HUMAN ADENOVIRUS Credibility of Association with Recreational Water: Strongly Associated
6 Viruses This chapter summarises the evidence for viral illnesses acquired through ingestion or inhalation of water or contact with water during water-based recreation. The organisms that will be described are: adenovirus; coxsackievirus; echovirus; hepatitis A virus; and hepatitis E virus. The following information for each organism is presented: general description, health aspects, evidence for association with recreational waters and a conclusion summarising the weight of evidence. © World Health Organization (WHO). Water Recreation and Disease. Plausibility of Associated Infections: Acute Effects, Sequelae and Mortality by Kathy Pond. Published by IWA Publishing, London, UK. ISBN: 1843390663 192 Water Recreation and Disease HUMAN ADENOVIRUS Credibility of association with recreational water: Strongly associated I Organism Pathogen Human adenovirus Taxonomy Adenoviruses belong to the family Adenoviridae. There are four genera: Mastadenovirus, Aviadenovirus, Atadenovirus and Siadenovirus. At present 51 antigenic types of human adenoviruses have been described. Human adenoviruses have been classified into six groups (A–F) on the basis of their physical, chemical and biological properties (WHO 2004). Reservoir Humans. Adenoviruses are ubiquitous in the environment where contamination by human faeces or sewage has occurred. Distribution Adenoviruses have worldwide distribution. Characteristics An important feature of the adenovirus is that it has a DNA rather than an RNA genome. Portions of this viral DNA persist in host cells after viral replication has stopped as either a circular extra chromosome or by integration into the host DNA (Hogg 2000). This persistence may be important in the pathogenesis of the known sequelae of adenoviral infection that include Swyer-James syndrome, permanent airways obstruction, bronchiectasis, bronchiolitis obliterans, and steroid-resistant asthma (Becroft 1971; Tan et al.