Highly Selective Inhibition of IMPDH2 Provides the Basis Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

KO Kidney.Xlsx
Supplemental Table 18: Dietary Impact on the CGL KO Kidney Sulfhydrome DR/AL Accession Molecular Cysteine Spectral Protein Name Number Alternate ID Weight Residues Count Ratio P‐value Ig gamma‐2A chain C region, A allele P01863 (+1) Ighg 36 kDa 10 C 5.952 0.03767 Heterogeneous nuclear ribonucleoprotein M Q9D0E1 (+1) Hnrnpm 78 kDa 6 C 5.000 0.00595 Phospholipase D3 O35405 Pld3 54 kDa 8 C 4.167 0.04761 Ig kappa chain V‐V region L7 (Fragment) P01642 Gm10881 13 kDa 2 C 2.857 0.01232 UPF0160 protein MYG1, mitochondrial Q9JK81 Myg1 43 kDa 7 C 2.333 0.01613 Copper homeostasis protein cutC homolog Q9D8X1 Cutc 29 kDa 7 C 10.333 0.16419 Corticosteroid‐binding globulin Q06770 Serpina6 45 kDa 3 C 10.333 0.16419 28S ribosomal protein S22, mitochondrial Q9CXW2 Mrps22 41 kDa 2 C 7.333 0.3739 Isoform 3 of Agrin A2ASQ1‐3 Agrn 198 kDa 2 C 7.333 0.3739 3‐oxoacyl‐[acyl‐carrier‐protein] synthase, mitochondrial Q9D404 Oxsm 49 kDa 11 C 7.333 0.3739 Cordon‐bleu protein‐like 1 Q3UMF0 (+3)Cobll1 137 kDa 10 C 5.833 0.10658 ADP‐sugar pyrophosphatase Q9JKX6 Nudt5 24 kDa 5 C 4.167 0.15819 Complement C4‐B P01029 C4b 193 kDa 29 C 3.381 0.23959 Protein‐glutamine gamma‐glutamyltransferase 2 P21981 Tgm2 77 kDa 20 C 3.381 0.23959 Isochorismatase domain‐containing protein 1 Q91V64 Isoc1 32 kDa 5 C 3.333 0.10588 Serpin B8 O08800 Serpinb8 42 kDa 11 C 2.903 0.06902 Heterogeneous nuclear ribonucleoprotein A0 Q9CX86 Hnrnpa0 31 kDa 3 C 2.667 0.5461 Proteasome subunit beta type‐8 P28063 Psmb8 30 kDa 5 C 2.583 0.36848 Ig kappa chain V‐V region MOPC 149 P01636 12 kDa 2 C 2.583 0.36848 -

Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model
Downloaded from http://www.jimmunol.org/ by guest on September 25, 2021 T + is online at: average * The Journal of Immunology , 34 of which you can access for free at: 2016; 197:1477-1488; Prepublished online 1 July from submission to initial decision 4 weeks from acceptance to publication 2016; doi: 10.4049/jimmunol.1600589 http://www.jimmunol.org/content/197/4/1477 Molecular Profile of Tumor-Specific CD8 Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. Waugh, Sonia M. Leach, Brandon L. Moore, Tullia C. Bruno, Jonathan D. Buhrman and Jill E. Slansky J Immunol cites 95 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2016/07/01/jimmunol.160058 9.DCSupplemental This article http://www.jimmunol.org/content/197/4/1477.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 25, 2021. The Journal of Immunology Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. -

IMPDH2: a New Gene Associated with Dominant Juvenile-Onset Dystonia-Tremor Disorder
www.nature.com/ejhg BRIEF COMMUNICATION OPEN IMPDH2: a new gene associated with dominant juvenile-onset dystonia-tremor disorder 1,8 1,8 2 3 1,4 2 5 Anna Kuukasjärvi , Juan✉ C. Landoni , Jyrki Kaukonen , Mika Juhakoski , Mari Auranen , Tommi Torkkeli , Vidya Velagapudi and Anu Suomalainen 1,6,7 © The Author(s) 2021 The aetiology of dystonia disorders is complex, and next-generation sequencing has become a useful tool in elucidating the variable genetic background of these diseases. Here we report a deleterious heterozygous truncating variant in the inosine monophosphate dehydrogenasegene(IMPDH2) by whole-exome sequencing, co-segregating with a dominantly inherited dystonia-tremor disease in a large Finnish family. We show that the defect results in degradation of the gene product, causing IMPDH2 deficiency in patient cells. IMPDH2 is the first and rate-limiting enzyme in the de novo biosynthesis of guanine nucleotides, a dopamine synthetic pathway previously linked to childhood or adolescence-onset dystonia disorders. We report IMPDH2 as a new gene to the dystonia disease entity. The evidence underlines the important link between guanine metabolism, dopamine biosynthesis and dystonia. European Journal of Human Genetics; https://doi.org/10.1038/s41431-021-00939-1 INTRODUCTION The disease-onset was between 9 and 20 years of age. Table 1 Dystonias are rare movement disorders characterised by sustained or summarises the clinical presentations. intermittent muscle contractions causing abnormal, often repetitive, movements and/or postures. Dystonia can manifest as an isolated Case report symptom or combined with e.g. parkinsonism or myoclonus [1]. While Patient II-6 is a 46-year-old woman. -

Study Protocol
PROTOCOL SYNOPSIS A Multicentre, Randomised, Double-blind, Placebo-controlled, Phase 3 Study Evaluating the Efficacy and Safety of Two Doses of Anifrolumab in Adult Subjects with Active Systemic Lupus Erythematosus International Coordinating Investigator Study site(s) and number of subjects planned Approximately 450 subjects are planned at approximately 173 sites. Study period Phase of development Estimated date of first subject enrolled Q2 2015 3 Estimated date of last subject completed Q2 2018 Study design This is a Phase 3, multicentre, multinational, randomised, double-blind, placebo-controlled study to evaluate the efficacy and safety of an intravenous treatment regimen of anifrolumab (150 mg or 300 mg) versus placebo in subjects with moderately to severely active, autoantibody-positive systemic lupus erythematosus (SLE) while receiving standard of care (SOC) treatment. The study will be performed in adult subjects aged 18 to 70 years of age. Approximately 450 subjects receiving SOC treatment will be randomised in a 1:2:2 ratio to receive a fixed intravenous dose of 150 mg anifrolumab, 300 mg anifrolumab, or placebo every 4 weeks (Q4W) for a total of 13 doses (Week 0 to Week 48), with the primary endpoint evaluated at the Week 52 visit. Investigational product will be administered as an intravenous (IV) infusion via an infusion pump over a minimum of 30 minutes, Q4W. Subjects must be taking either 1 or any combination of the following: oral corticosteroids (OCS), antimalarial, and/or immunosuppressants. Randomisation will be stratified using the following factors: SLE Disease Activity Index 2000 (SLEDAI-2K) score at screening (<10 points versus ≥10 points); Week 0 (Day 1) OCS dose 2(125) Revised Clinical Study Protocol Drug Substance Anifrolumab (MEDI-546) Study Code D3461C00005 Edition Number 5 Date 18 May 2016 (<10 mg/day versus ≥10 mg/day prednisone or equivalent); and results of a type 1 interferon (IFN) test (high versus low). -

WO2021044381A1.Pdf
( (51) International Patent Classification: KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, G01N 33/543 (2006.01) COIN 21/00 (2006.01) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, (21) International Application Number: SA, SC, SD, SE, SG, SK, SL, ST, SV, SY, TH, TJ, TM, TN, PCT/IB2020/058284 TR, TT, TZ, UA, UG, US, UZ, VC, VN, WS, ZA, ZM, ZW. (22) International Filing Date: (84) Designated States (unless otherwise indicated, for every 04 September 2020 (04.09.2020) kind of regional protection available) . ARIPO (BW, GH, (25) Filing Language: English GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (26) Publication Language: English TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (30) Priority Data: EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, 62/897,042 06 September 2019 (06.09.2019) US MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, TR), OAPI (BF, BJ, CF, CG, Cl, CM, GA, GN, GQ, GW, (72) Inventors; and KM, ML, MR, NE, SN, TD, TG). (71) Applicants: GORI, Alessandro [IT/IT]; Via Murri 23, 20871 Vimercate (IT). CRETICH, Marina [IT/IT]; Via Published: Zurigo 20, 20147 Milano (IT). CHIARI, Marcella [IT/IT]; — with international search report (Art. 21(3)) Via Gian Battista Brochi 11, 2013 1 Milano (IT). -

Chuanxiong Rhizoma Compound on HIF-VEGF Pathway and Cerebral Ischemia-Reperfusion Injury’S Biological Network Based on Systematic Pharmacology
ORIGINAL RESEARCH published: 25 June 2021 doi: 10.3389/fphar.2021.601846 Exploring the Regulatory Mechanism of Hedysarum Multijugum Maxim.-Chuanxiong Rhizoma Compound on HIF-VEGF Pathway and Cerebral Ischemia-Reperfusion Injury’s Biological Network Based on Systematic Pharmacology Kailin Yang 1†, Liuting Zeng 1†, Anqi Ge 2†, Yi Chen 1†, Shanshan Wang 1†, Xiaofei Zhu 1,3† and Jinwen Ge 1,4* Edited by: 1 Takashi Sato, Key Laboratory of Hunan Province for Integrated Traditional Chinese and Western Medicine on Prevention and Treatment of 2 Tokyo University of Pharmacy and Life Cardio-Cerebral Diseases, Hunan University of Chinese Medicine, Changsha, China, Galactophore Department, The First 3 Sciences, Japan Hospital of Hunan University of Chinese Medicine, Changsha, China, School of Graduate, Central South University, Changsha, China, 4Shaoyang University, Shaoyang, China Reviewed by: Hui Zhao, Capital Medical University, China Background: Clinical research found that Hedysarum Multijugum Maxim.-Chuanxiong Maria Luisa Del Moral, fi University of Jaén, Spain Rhizoma Compound (HCC) has de nite curative effect on cerebral ischemic diseases, *Correspondence: such as ischemic stroke and cerebral ischemia-reperfusion injury (CIR). However, its Jinwen Ge mechanism for treating cerebral ischemia is still not fully explained. [email protected] †These authors share first authorship Methods: The traditional Chinese medicine related database were utilized to obtain the components of HCC. The Pharmmapper were used to predict HCC’s potential targets. Specialty section: The CIR genes were obtained from Genecards and OMIM and the protein-protein This article was submitted to interaction (PPI) data of HCC’s targets and IS genes were obtained from String Ethnopharmacology, a section of the journal database. -

Polymorphisms of the Multidrug Pump ABCG2: a Systematic Review of Their Effect on Protein Expression, Function, and Drug Pharmacokinetics
1521-009X/46/12/1886–1899$35.00 https://doi.org/10.1124/dmd.118.083030 DRUG METABOLISM AND DISPOSITION Drug Metab Dispos 46:1886–1899, December 2018 Copyright ª 2018 by The American Society for Pharmacology and Experimental Therapeutics Minireview Polymorphisms of the Multidrug Pump ABCG2: A Systematic Review of Their Effect on Protein Expression, Function, and Drug Pharmacokinetics Niall Heyes, Parth Kapoor, and Ian D. Kerr School of Life Sciences, Queen’s Medical Centre, University of Nottingham, Nottingham, United Kingdom Received June 12, 2018; accepted September 20, 2018 Downloaded from ABSTRACT The widespread expression and polyspecificity of the multidrug to improve outcomes in cancer patients treated with tyrosine ABCG2 efflux transporter make it an important determinant of the kinase inhibitors, but the reasons for this are yet to be estab- pharmacokinetics of a variety of substrate drugs. Null ABCG2 lished, and this residue’s role in the mechanism of the protein is expression has been linked to the Junior blood group. Polymor- unexplored by current biochemical and structural approaches. phisms affecting the expression or function of ABCG2 may have Research into the less-common polymorphisms is confined to dmd.aspetjournals.org clinically important roles in drug disposition and efficacy. The in vitro studies, with several polymorphisms shown to decrease most well-studied single nucleotide polymorphism (SNP), Q141K resistance to anticancer agents such as SN-38 and mitoxantrone. (421C>A), is shown to decrease ABCG2 expression and activity, In this review, we present a systematic analysis of the effects of resulting in increased total drug exposure and decreased re- ABCG2 polymorphisms on ABCG2 function and drug pharmaco- sistance to various substrates. -

IMPDH Inhibitors for Anti-Tumor Therapy in Tuberous Sclerosis Complex
bioRxiv preprint doi: https://doi.org/10.1101/835199; this version posted November 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. IMPDH inhibitors for anti-tumor therapy in tuberous sclerosis complex Alexander J. Valvezan1, *, Spencer K. Miller1, Molly C. McNamara1, Margaret E. Torrence1, John M. Asara2, Elizabeth P. Henske3, Brendan D. Manning1,* 1Department of Genetics and Complex Diseases, Harvard T.H. Chan School of Public Health, Boston, MA, USA 2Division of Signal Transduction, Beth Israel Deaconess Medical Center, and Department of Medicine, Harvard Medical School, Boston, MA, USA 3Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA *Correspondence to: [email protected] or [email protected] 665 Huntington Ave, SPH2-117 Boston, MA 02115 617 432-5614 Running title: Repurposing mizoribine for tuberous sclerosis complex tumors Keywords: mTORC1, IMPDH, mizoribine, mycophenolate, tuberous sclerosis complex Financial support: This research was supported by grants from the National Institutes of Health (P01CA120964 to J.M.A. E.P.H. and B.D.M., P30CA006516 to J.M.A., and R35CA197459 to B.D.M.) and a Rothberg Courage Award from the TS Alliance to B.D.M. Conflict of interest statement: B.D.M. is a shareholder and scientific advisory board member for Navitor Pharmaceuticals and LAM Therapeutics. Word count: 5,171 Number of figures: 6 main figures, 4 supplemental figures 1 bioRxiv preprint doi: https://doi.org/10.1101/835199; this version posted November 11, 2019. -

Efficacy and Safety of Mizoribine by One Single Dose Administration for Patients with Rheumatoid Arthritis
□ ORIGINAL ARTICLE □ Efficacy and Safety of Mizoribine by One Single Dose Administration for Patients with Rheumatoid Arthritis Kunihiro Ichinose 1, Tomoki Origuchi 2, Shin-ya Kawashiri 1, Naoki Iwamoto 1, Keita Fujikawa 1, Toshiyuki Aramaki 1, Makoto Kamachi 1, Kazuhiko Arima 1, Mami Tamai 1, Hideki Nakamura 1, Hiroaki Ida 1, Atsushi Kawakami 1, Toshiaki Tsukada 3, Yukitaka Ueki 4 and Katsumi Eguchi 1 Abstract Objective Mizoribine (MZR) is an immunosuppressant that inhibits nucleic acid metabolism and is a rela- tively safe disease-modifying anti-rheumatic drug (DMARD). We evaluated the efficacy and safety of one single dose per day for patients with rheumatoid arthritis (RA). Patients and Methods In this study 32 patients with RA received MZR therapy. We evaluated the average dose of MZR and prednisolone, response to treatment and peak plasma level of MZR. Results The average dose of MZR was 146.1±31.2 (range: 50-200) mg/day. The average dose of predniso- lone was 4.63±3.59 (range: 0-14) mg/day. The average plasma level of MZR, measured after 3 hours, was 2.20±0.49 μg/mL in the responder group and 1.59±0.82 μg/mL in the non-responder group (p=0.020). The treatment with MZR for 24 weeks was completed by 71.9% of patients and the proportion of patients who achieved a good and moderate response rate according to the European League Against Rheumatism (EU- LAR) criteria was 56.3% at 24 weeks. The plasma level of MZR which was greater than or equal to 2.12 μg/ mL was significantly correlated with the clinical response (p<0.01). -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -
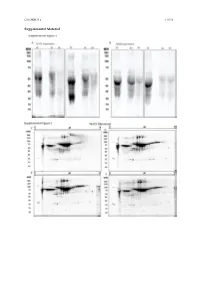
Type of the Paper (Article
Cells 2020, 9, x 1 of 19 Supplemental Material Cells 2020, 9, x 2 of 19 Figure S1. Secretome enrichment: protocol optimization. 1D SDS-PAGE documentation of washing steps: Culture medium was substituted with FCS-free medium, which was changed every 2 h. The supernatants were then collected, and the proteins isolated and separated in 1D SDS-PAGE ((A) TK173 and (B) TK188). Proteins were stained with Flamingo fluorescent gel stain. Two-dimensional pattern of the proteins isolated from supernatant of TK173, (C) 2 h, (D) 4 h, (E) 6 h, and (F) 8 h after changing to FCS-free medium. (G) Cell secretome collected 24 h after elimination of the contaminating FCS-proteins with different washing steps. Proteins were stained with Flamingo fluorescent gel stain. Cells 2020, 9, x 3 of 19 Figure S2. 2-DE reference maps of secretomes; 150 μg proteins were loaded on an 11 cm IPG strip with a linear pH gradient PI 5–8 for IEF; 12% SDS-polyacrylamide gels were used for the second dimension. Proteins were stained with Flamingo fluorescent gel stain. Identified spots were assigned a number corresponding to that in their table. 2-DE maps from secretome of (A) TK173 control and (B) TGFβ1- treated ones. The 2-DE patterns revealed an alteration of secretome in stimulated TK173. Secretome patterns from TK173 treated with (C) ANG II and (D) PDGF. Cells 2020, 9, x 4 of 19 A Figure S3. Classification of the differentially expressed proteins upon ANG II, TGFβ1, or PDGF treatment in TK173. (A) Bar charts of the cellular component analyzed by STRAP biological function analysis in which the identified proteins from all treatments in both cell types are involved. -

Fluorescent Probes for Nucleic Acid Visualization in Fixed and Live Cells
Molecules 2013, 18, 15357-15397; doi:10.3390/molecules181215357 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Fluorescent Probes for Nucleic Acid Visualization in Fixed and Live Cells Alexandre S. Boutorine 1,*, Darya S. Novopashina 2, Olga A. Krasheninina 2,3, Karine Nozeret 1 and Alya G. Venyaminova 2 1 Muséum National d’Histoire Naturelle, CNRS, UMR 7196, INSERM, U565, 57 rue Cuvier, B.P. 26, Paris Cedex 05, F-75231, France; E-Mail: [email protected] 2 Institute of Chemical Biology and Fundamental Medicine, Siberian Division of Russian Academy of Sciences, Lavrentyev Ave., 8, Novosibirsk 630090, Russia; E-Mails: [email protected] (D.S.N.); [email protected] (O.A.K.); [email protected] (A.G.V.) 3 Department of Natural Sciences, Novosibirsk State University, Pirogova Str., 2, Novosibirsk 630090, Russia * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +33-1-40-79-36-96; Fax: +33-1-40-79-37-05. Received: 23 September 2013; in revised form: 20 November 2013 / Accepted: 5 December 2013 / Published: 11 December 2013 Abstract: This review analyses the literature concerning non-fluorescent and fluorescent probes for nucleic acid imaging in fixed and living cells from the point of view of their suitability for imaging intracellular native RNA and DNA. Attention is mainly paid to fluorescent probes for fluorescence microscopy imaging. Requirements for the target-binding part and the fluorophore making up the probe are formulated. In the case of native double-stranded DNA, structure-specific and sequence-specific probes are discussed.