Snail Mucus from the Mantle and Foot of Two Land
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shell Morphology, Radula and Genital Structures of New Invasive Giant African Land
bioRxiv preprint doi: https://doi.org/10.1101/2019.12.16.877977; this version posted December 16, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Shell Morphology, Radula and Genital Structures of New Invasive Giant African Land 2 Snail Species, Achatina fulica Bowdich, 1822,Achatina albopicta E.A. Smith (1878) and 3 Achatina reticulata Pfeiffer 1845 (Gastropoda:Achatinidae) in Southwest Nigeria 4 5 6 7 8 9 Alexander B. Odaibo1 and Suraj O. Olayinka2 10 11 1,2Department of Zoology, University of Ibadan, Ibadan, Nigeria 12 13 Corresponding author: Alexander B. Odaibo 14 E.mail :[email protected] (AB) 15 16 17 18 1 bioRxiv preprint doi: https://doi.org/10.1101/2019.12.16.877977; this version posted December 16, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 19 Abstract 20 The aim of this study was to determine the differences in the shell, radula and genital 21 structures of 3 new invasive species, Achatina fulica Bowdich, 1822,Achatina albopicta E.A. 22 Smith (1878) and Achatina reticulata Pfeiffer, 1845 collected from southwestern Nigeria and to 23 determine features that would be of importance in the identification of these invasive species in 24 Nigeria. -

137-144. New Hemiplecta
Biodiversity Journal , 2012, 3 (2): 137-144 A new species of Hemiplecta Albers, 1850 (Gastropoda, Pul - monata, Ariophantidae) from Sumatra, Indonesia David P. Cilia 1* & John Abbas 2 1 29, Triq il-Palazz l-Aħmar, Santa Venera, Malta 28, Jalan Demaga Baru, Muara Angke, Jakarta Utara Pos 14450, Jakarta, Indonesia *Corresponding author, e-mail: [email protected] ABSTRACT The ariophantid Hemiplecta belerang sp. nov. from South Sumatra is described in this paper. It is compared with its closest congeners, from which it is geographically and reproductively isolated. KEY WORDS Ariophantidae; Hemiplecta belerang n. sp.; Sumatra; Indonesia. Received 03.06.2012; accepted 21.06.2012; printed 30.06.2012 INTRODUCTION d'Histoire Naturelle, Paris, France (MNHN); Na - tural History Museum, London, United Kingdom The family Ariophantidae Godwin-Austen, (NHMUK); National Museum of Natural History, 1888 is nested within the limacoid clade of pulmo - Mdina, Malta (NMNH); Zoological Department nates and is native to south-east Asia and India of Tel Aviv University, Israel (TAU); Institut für (Hausdorf, 2000). Evolutionsbiologie und Umweltwissenschaften/ The family includes the genus Hemiplecta Al - Zoologisches Museum Universität Zürich-Irchel, bers, 1850, a group of medium to large-sized Switzerland (ZMZ). ground-inhabiting snails (Boonngam et al., 2008; Morphology and anatomy. DG = dart gland; Schilthuizen, 2008), and the Sumatran representa - DGR = dart gland retractor muscle; D = diameter; tives include H. abbasi Maassen, 2009, H. goliath E = epiphallus; EC = epiphallic caecum; F = fla - van Benthem Jutting, 1959, H. humphreysiana gellum; GA = genital atrium; H = height; ht = ho - (Lea, 1840), H. obliquata (Reeve, 1852) and H. lotype; P = penis; PRM = penial retractor muscle; obliqueundulata van Benthem Jutting, 1959 (see S = spermatheca; sd = standard deviation; U = um - van Benthem Jutting, 1959; Dharma, 2005; Maas - bilicus; V = vagina; VD = was deferens; x = mean sen, 2009). -

The Malacological Society of London
ACKNOWLEDGMENTS This meeting was made possible due to generous contributions from the following individuals and organizations: Unitas Malacologica The program committee: The American Malacological Society Lynn Bonomo, Samantha Donohoo, The Western Society of Malacologists Kelly Larkin, Emily Otstott, Lisa Paggeot David and Dixie Lindberg California Academy of Sciences Andrew Jepsen, Nick Colin The Company of Biologists. Robert Sussman, Allan Tina The American Genetics Association. Meg Burke, Katherine Piatek The Malacological Society of London The organizing committee: Pat Krug, David Lindberg, Julia Sigwart and Ellen Strong THE MALACOLOGICAL SOCIETY OF LONDON 1 SCHEDULE SUNDAY 11 AUGUST, 2019 (Asilomar Conference Center, Pacific Grove, CA) 2:00-6:00 pm Registration - Merrill Hall 10:30 am-12:00 pm Unitas Malacologica Council Meeting - Merrill Hall 1:30-3:30 pm Western Society of Malacologists Council Meeting Merrill Hall 3:30-5:30 American Malacological Society Council Meeting Merrill Hall MONDAY 12 AUGUST, 2019 (Asilomar Conference Center, Pacific Grove, CA) 7:30-8:30 am Breakfast - Crocker Dining Hall 8:30-11:30 Registration - Merrill Hall 8:30 am Welcome and Opening Session –Terry Gosliner - Merrill Hall Plenary Session: The Future of Molluscan Research - Merrill Hall 9:00 am - Genomics and the Future of Tropical Marine Ecosystems - Mónica Medina, Pennsylvania State University 9:45 am - Our New Understanding of Dead-shell Assemblages: A Powerful Tool for Deciphering Human Impacts - Sue Kidwell, University of Chicago 2 10:30-10:45 -

An Assessment of Land and Aquatic Snails in the South African Pet Trade
Management of Biological Invasions (2020) Volume 11, Issue 3: 512–524 CORRECTED PROOF Research Article Exotic gastropods for sale: an assessment of land and aquatic snails in the South African pet trade Tinyiko C. Shivambu, Ndivhuwo Shivambu and Colleen T. Downs* Centre for Excellence in Invasion Biology, and Centre for Functional Biodiversity, School of Life Sciences, University of KwaZulu-Natal, Private Bag X01, Scottsville, Pietermaritzburg, 3209, South Africa Author e-mails: [email protected] (CTD), [email protected] (TCS), [email protected] (NS) *Corresponding author Citation: Shivambu TC, Shivambu N, Downs CT (2020) Exotic gastropods for Abstract sale: an assessment of land and aquatic snails in the South African pet trade. Gastropods are amongst the most popular of the Mollusca in the pet trade, with Management of Biological Invasions 11(3): globalisation being the main contributing factor facilitating their establishment 512–524, https://doi.org/10.3391/mbi.2020.11.3.11 globally. Although it is known that gastropods are kept as pets in South Africa, Received: 27 February 2020 relatively little has been documented on the trade for this group. Physical pet stores Accepted: 20 May 2020 selling gastropod species were surveyed seasonally in South Africa, aiming to determine 1) the types of species sold, including their trade popularity, trade volume, Published: 1 August 2020 and the biogeographic realms they originated from, and 2) seasonal variations in Handling editor: Ana Novoa gastropod species traded. Six gastropod species were recorded in the South African Thematic editor: Catherine Jarnevich pet stores with three known invasives (Achatina fulica, A. immaculata, and Pomacea Copyright: © Shivambu et al. -

Abstract Volume
ABSTRACT VOLUME August 11-16, 2019 1 2 Table of Contents Pages Acknowledgements……………………………………………………………………………………………...1 Abstracts Symposia and Contributed talks……………………….……………………………………………3-225 Poster Presentations…………………………………………………………………………………226-291 3 Venom Evolution of West African Cone Snails (Gastropoda: Conidae) Samuel Abalde*1, Manuel J. Tenorio2, Carlos M. L. Afonso3, and Rafael Zardoya1 1Museo Nacional de Ciencias Naturales (MNCN-CSIC), Departamento de Biodiversidad y Biologia Evolutiva 2Universidad de Cadiz, Departamento CMIM y Química Inorgánica – Instituto de Biomoléculas (INBIO) 3Universidade do Algarve, Centre of Marine Sciences (CCMAR) Cone snails form one of the most diverse families of marine animals, including more than 900 species classified into almost ninety different (sub)genera. Conids are well known for being active predators on worms, fishes, and even other snails. Cones are venomous gastropods, meaning that they use a sophisticated cocktail of hundreds of toxins, named conotoxins, to subdue their prey. Although this venom has been studied for decades, most of the effort has been focused on Indo-Pacific species. Thus far, Atlantic species have received little attention despite recent radiations have led to a hotspot of diversity in West Africa, with high levels of endemic species. In fact, the Atlantic Chelyconus ermineus is thought to represent an adaptation to piscivory independent from the Indo-Pacific species and is, therefore, key to understanding the basis of this diet specialization. We studied the transcriptomes of the venom gland of three individuals of C. ermineus. The venom repertoire of this species included more than 300 conotoxin precursors, which could be ascribed to 33 known and 22 new (unassigned) protein superfamilies, respectively. Most abundant superfamilies were T, W, O1, M, O2, and Z, accounting for 57% of all detected diversity. -

The Diversity of Land Snail Fauna in Chonburi Province, Eastern Thailand
Kasetsart J. (Nat. Sci.) 42 : 256 - 263 (2008) The Diversity of Land Snail Fauna in Chonburi Province, Eastern Thailand Pratin Boonngam*, Pongrat Dumrongrojwattana and Surin Matchacheep ABSTRACT Land snails diversity were investigated in several habitats in Chonburi Province, Eastern Thailand. Snails were collected from 14 areas throughout Chonburi Province. A total of 16 families 29 genera and 48 species were recorded, 22 of which had been previously reported. The others could be identified into genus level and at least nine of them are being proposed as new to science. Key words: land snail, Gastropoda, Chonburi province INTRODUCTION snail were reported for Chonburi Province previously by Panha (1996) Hemmen and Land snails belong to the Phylum Hemmen (2001) Panha and Burch (2005). The Mollusca, Class Gastropoda and include two present study was undertaken to update the species groups prosobranchs and pulmonates. list of land snails in Chonburi Province. Prosobranchs, frequently have heavily calcified shells and opercula covering the aperture or MATERIALS AND METHODS opening or their shells. Pulmonates, lack opercula and used lung in gas exchange process. As Specimens were collected from several herbivores, snails eat many kinds of fresh and dead habitats in Chonburi Province. Methods consisted leaves and are eaten by many animals such as some of collecting soil samples where shells or predacious insects, snakes, birds and small fragments of shells were found, or where snails mammals. They live under leaves, litter, logs, were suspected to be present. Limestone soils are stones and trash. Some land snails carry serve as by far the richest source of snails in Chonburi hosts for some parasites such as Hemiplecta Province. -

First Chromosome Analysis and Localization of the Nucleolar Organizer Region of Land Snail, Sarika Resplendens (Stylommatophora, Ariophantidae) in Thailand
© 2013 The Japan Mendel Society Cytologia 78(3): 213–222 First Chromosome Analysis and Localization of the Nucleolar Organizer Region of Land Snail, Sarika resplendens (Stylommatophora, Ariophantidae) in Thailand Wilailuk Khrueanet1, Weerayuth Supiwong2, Chanidaporn Tumpeesuwan3, Sakboworn Tumpeesuwan3, Krit Pinthong4, and Alongklod Tanomtong2* 1 School of Science and Technology, Khon Kaen University, Nong Khai Campus, Muang, Nong Khai 43000, Thailand 2 Applied Taxonomic Research Center (ATRC), Department of Biology, Faculty of Science, Khon Kaen University, Muang, Khon Kaen 40002, Thailand 3 Department of Biology, Faculty of Science, Mahasarakham University, Kantarawichai, Maha Sarakham 44150, Thailand 4 Biology Program, Faculty of Science and Technology, Surindra Rajabhat University, Muang, Surin 32000, Thailand Received July 23, 2012; accepted February 25, 2013 Summary We report the first chromosome analysis and localization of the nucleolar organizer re- gion of the land snail Sarika resplendens (Philippi 1846) in Thailand. The mitotic and meiotic chro- mosome preparations were carried out by directly taking samples from the ovotestis. Conventional and Ag-NOR staining techniques were applied to stain the chromosomes. The results showed that the diploid chromosome number of S. resplendens is 2n=66 and the fundamental number (NF) is 132. The karyotype has the presence of six large metacentric, two large submetacentric, 26 medium metacentric, and 32 small metacentric chromosomes. After using the Ag-NOR banding technique, one pair of nucleolar organizer regions (NORs) was observed on the long arm subtelomeric region of chromosome pair 11. We found that during metaphase I, the homologous chromosomes show synapsis, which can be defined as the formation of 33 ring bivalents, and 33 haploid chromosomes at metaphase II as diploid species. -
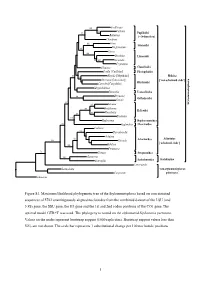
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia -

Zoologische Mededelingen 78-02
Comparative reproductive anatomy in the South African giant land snails (Gastropoda: Pulmonata: Achatinidae) A.R. Mead Mead, A.R. Comparative reproductive anatomy in the South African giant land snails (Gastropoda: Pulmonata: Achatinidae). Zool. Med. Leiden 78 (25), 31.xii.2004: 417-449, figs 1-39.— ISSN 0024-0672. A.R. Mead, Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, Ari- zona 85721, U.S.A. (e-mail: [email protected]). Key words: Mollusca; Gastropoda; Pulmonata; Achatinidae; biogeography; taxonomy; genital anatomy; Southern Africa; East Africa. The history and current taxonomic status of 62 nominal taxa are revised that have been associated in the literature with the subgenus Tholachatina Bequaert, 1950, of genus Archachatina Albers, 1850, and the genus Cochlitoma Férussac, 1821, in the land snail family Achatinidae Swainson, 1840. Tangible, reliable characters have been found in the detailed features of the reproductive anatomy in this family. The results of comparative anatomical study convincingly reflect phylogeny in contrast to the comparative study of only the shell characters. This latter more strongly reflects the effects of the intrinsically variable environment over time. In the present study, both sets of characters are needed to refine identification. Change, and therefore speciation, is shown in the reproductive system through anatomical differ- ences that may develop in the functional interrelationships of the two integral reproductive systems of hermaphroditism. Limited adjustment to anatomical change over time has established for each genus a typical, characteristic reproductive anatomical pattern or configuration. Because this pat- tern has a basically high degree of physical stability within a population, it becomes an identifying character for the genus, and more restrictedly so for the species. -

(GISD) 2021. Species Profile Achatina Fulica. Available From
FULL ACCOUNT FOR: Achatina fulica Achatina fulica System: Terrestrial Kingdom Phylum Class Order Family Animalia Mollusca Gastropoda Stylommatophora Achatinidae Common name Afrikanische Riesenschnecke (German), giant African snail (English), giant African land snail (English) Synonym Lissachatina fulica , (Bowdich 1822) Similar species Summary Achatina fulica feeds on a wide variety of crop plants and may present a threat to local flora. Populations of this pest often crash over time (20 to 60 years) and this should not be percieved as effectiveness of the rosy wolfsnail (Euglandina rosea) as a biocontrol agent. Natural chemicals from the fruit of Thevetia peruviana have activity against A. fulica and the cuttings of the alligator apple (Annona glabra) can be used as repellent hedges against A. fulica. view this species on IUCN Red List Species Description Achatina fulica has a narrow, conical shell, which is twice as long as it is wide and contains 7 to 9 whorls when fully grown. The shell is generally reddish-brown in colour with weak yellowish vertical markings but colouration varies with environmental conditions and diet. A light coffee colour is common. Adults of the species may exceed 20cm in shell length but generally average about 5 to 10cm. The average weight of the snail is approximately 32 grams (Cooling 2005). Please see PaDIL (Pests and Diseases Image Library) Species Content Page Non-insects Giant African Snail for high quality diagnostic and overview images. Global Invasive Species Database (GISD) 2021. Species profile Achatina fulica. Pag. 1 Available from: http://www.iucngisd.org/gisd/species.php?sc=64 [Accessed 08 October 2021] FULL ACCOUNT FOR: Achatina fulica Notes The Achatinidae gastropod family is native to Africa. -

A New Hemiplecta Species from a Remote Mountain in South-East Sumatra, Indonesia (Gastropoda, Pulmonata, Ariophantidae)
Basteria73(1-3)-TOTAAL:Basteria-basis.qxd 05/10/2009 23:37 Page 77 BASTERIA, 73: 77-80, 2009 A new Hemiplecta species from a remote mountain in south-east Sumatra, Indonesia (Gastropoda, Pulmonata, Ariophantidae) Wim J.M. MAASSEN Nationaal Natuurhistorisch Museum Naturalis, P.O. Box 9517, NL 2300 RA Leiden, The Netherlands; [email protected] Hemiplecta abbasi spec. nov. is described from SE Sumatra, Indonesia. Key words: Mollusca, Gastropoda, Pulmonata, Ariophantidae, Hemiplecta, taxonomy, South East Asia, Indonesia, Sumatra. INTRODUCTION A sample, containing some specimens of a supposed Hemiplecta species, collected by Mr. J. Abbas (Jakarta) in south-east Sumatra, was received for identification. Thanks to the publications of Van Benthem Jutting (1950a, b, 1959) on the non-marine molluscs from Sumatra and Java, the malacofauna of these two islands is relatively well known, espe- cially with regard to the larger species. In her “Catalogue of the non-marine Mollusca of Sumatra” Van Benthem Jutting (1959) not only listed all the species, but also discussed the records for Sumatra. Therefore it was made easier to conclude that this Hemiplecta sample must represent not only a new record but even a new species, which is described below. Abbreviations. – For shell characters: W, width; H, height. For anatomical characters: DS, dart sac; EC, epiphallic caecum; EP, epiphallus; FL, flagellum; GA, glandula albu- minifera; O, oviductus; P, penis; SD, spermathecal duct; SO, spermoviductus; SP, sper- matheca; R, retractor muscle; V, vagina; VD, vas deferens. For collections: MZB, Museum Zoologicum Bogoriense, Bogor, Java, Indonesia; RMNH, Nationaal Natuurhistorisch Museum Naturalis, Leiden, The Netherlands; MD, reference collection W.J.M. -

University of Florida Thesis Or Dissertation Formatting
EFFECTS OF DIET AND DENSITY ON Lissachatina fulica (STYLOMMATOPHORA: ACHATINIDAE) By KATRINA L. DICKENS A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2016 © 2016 Katrina L. Dickens To Theodora Dickens ACKNOWLEDGMENTS I would like to thank my advisor, Dr. John Capinera, for his mentoring, feedback and advice. I also thank Dr. Trevor Smith for acting as my committee member and for giving me the opportunity to do this research. Also I thank all of the workers at the Department of Agriculture and Consumer Services, Division of Plant Industry in Gainesville, Florida that helped in this research and colony maintenance, specifically Cory Penca, Amy Howe, Jessica McGuire, Shannen Leahy, Shweta Sharma, Addison Mertz, Cason Bartz, and Steven Rowley. 4 TABLE OF CONTENTS page ACKNOWLEDGMENTS .................................................................................................. 4 LIST OF TABLES ............................................................................................................ 7 LIST OF FIGURES .......................................................................................................... 8 ABSTRACT ..................................................................................................................... 9 CHAPTER 1 LITERATURE REVIEW .......................................................................................... 11 Agricultural Pest .....................................................................................................