Modifications of Polyaspartimide and Poly(Ethylene-Co-Methacrylic Acid) :: Investigation of Potential Fungicidal Support Polymers in a Nylon 6 Blend/ Carl J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acid-Base Properties, Deactivation, and in Situ Regeneration of Condensation Catalysts for Synthesis of Methyl Methacrylate
ACID-BASE PROPERTIES, DEACTIVATION, AND IN SITU REGENERATION OF CONDENSATION CATALYSTS FOR SYNTHESIS OF METHYL METHACRYLATE MAKARAND R. GOGATE', JAMES J. SPIVEY', AND JOSEPH R. ZOELLER2 'Research Triangle Institute, 3040 Cornwallis Road, RTP, NC 27709-2 194 2Eastman Chemical Company, Kingsport, TN, 37662-5 150 ABSTRACT Condensation reaction of a propionate with formaldehyde is a novel route for synthesis of methyl methacrylate (MMA). The reaction mechanism involves a proton abstraction from the propionate on the basic sites and activation of the aliphatic aldehyde on the acidic sites of the catalyst. The acid-base properties of ternary V-Si-P oxide catalysts and their relation to the MMA yield in the vapor phase condensation of formaldehyde with propionic anhydride has been studied for the first time. Five different V-Si-P catalysts with different atomic ratios of vanadium, silicon, and phosphorous were synthesized, characterized, and tested in a fixed-bed microreactor system. A V-Si-P 1:10:2.8 catalyst gave the maximum condensation yield of 56% based on HCHO fed at 300 "C and 2 atm and at a space velocity of 290 cc/g cateh. A parameter called the "q-ratio" has been defined to correlate the condensation yields to the acid-base properties. The correlation of q-ratio with the condensation yield shows that higher q-ratios are more desirable. The long-term deactivation studies on the V-Si-P 1: 10:2.8 catalyst at 300 "C and 2 atm and at a space velocity of 290 cc/g cat-h show that the catalyst activity drops by a factor of nearly 20 over a 180 h period. -

Polymer Dynamics
Polymer Dynamics 2017 School on Soft Matters and Biophysics Instructor: Alexei P. Sokolov, e-mail: [email protected] Text: Instructor's notes with supplemental reading from current texts and journal articles. Please, have the notes printed out before each lecture. Books (optional): M.Doi, S.F.Edwards, “The Theory of Polymer Dynamics”. Y.Grosberg, A.Khokhlov, “Statistical Physics of Macromolecules”. J.Higgins, H.Benoit, “Polymers and Neutron Scattering”. 1 Course Content: I. Introduction II. Experimental methods for analysis of molecular motions III. Vibrations IV. Fast and Secondary Relaxations V. Segmental Dynamics and Glass Transition VI. Chain Dynamics and Viscoelastic Properties VII. Rubber Elasticity VIII. Concluding Remarks 2 INTRODUCTION Polymers are actively used in many technologies Traditional technologies: Even better perspectives for use in novel technologies: Example: Light-weight materials Boeing-787 “Dreamliner” 80% by volume is plastic Materials for energy generation Example: polymer solar cells Materials for energy storage Example: Polymer-based Li battery Polymers have huge potential for applications in Bio-medical field “Smart” materials, stimuli-responsive, self-healing … 3 Polymers Polymers are long molecules They are constructed by covalently bonded structural units – monomers Main difference between properties of small molecules and polymers is related to the chain connectivity Advantages of Polymeric Based Materials: Easy processing, relatively cheap manufacturing Light weight (contain mostly light elements like C, H, O) Unique viscoelastic properties (e.g. rubber elasticity) Extremely broad tunability of macroscopic properties 4 Definitions o Monomer – repeat unit, structural block of the polymer chain o Oligomer – short chains, ~ 3-10 monomers o Polymers – long chains, usually hundreds of monomers o Degree of polymerization – number of monomers in the polymer chain Molecular weight: Weight of the molecule M [g/mol]. -

Submitted To
I 1 RTI PROJECT NO. 9611-6048 DOE Contract No. DE-AC22-94PC94065 January 1,1995 through March 31,1995 SYNTHESIS OF ACRYLATES AND METHACRLYATES FROM COAL-DERIVED SYNGAS Quarterly Technical Progress Report Submitted to US. Department of Energy Pittsburgh Energy Technology Center P. 0. Box 10940 Pittsburgh, Pennsylvania 15236-0940 Submitted by Research Triangle Institute P. 0. Box 12194 Research Triangle Park, NC 27709 DOE COR: Richard E. Tischer RTI Project Manager: James J. Spivey DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsi- bility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Refer- ence herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recom- mendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the DiCJTRlBfllQN THIS DOWMENT mm United States Government or any agency thereof. OF %”/ - DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best available original document. Executive Summary Task 1-Synthesis of Propionates The objective of this Task is to develop the technology for the synthesis of low-cost propionates. -

Biodegradable Polymeric Biomaterials in Different Forms for Long-Acting
University of Tennessee Health Science Center UTHSC Digital Commons Theses and Dissertations (ETD) College of Graduate Health Sciences 5-2017 Biodegradable Polymeric Biomaterials in Different Forms for Long-acting Contraception and Drug Delivery to the Eye and Brain Dileep Reddy Janagam University of Tennessee Health Science Center Follow this and additional works at: https://dc.uthsc.edu/dissertations Part of the Pharmaceutics and Drug Design Commons Recommended Citation Janagam, Dileep Reddy (http://orcid.org/0000-0002-7235-7709), "Biodegradable Polymeric Biomaterials in Different Forms for Long-acting Contraception and Drug Delivery to the Eye and Brain" (2017). Theses and Dissertations (ETD). Paper 425. http://dx.doi.org/10.21007/etd.cghs.2017.0429. This Dissertation is brought to you for free and open access by the College of Graduate Health Sciences at UTHSC Digital Commons. It has been accepted for inclusion in Theses and Dissertations (ETD) by an authorized administrator of UTHSC Digital Commons. For more information, please contact [email protected]. Biodegradable Polymeric Biomaterials in Different Forms for Long-acting Contraception and Drug Delivery to the Eye and Brain Document Type Dissertation Degree Name Doctor of Philosophy (PhD) Program Pharmaceutical Sciences Track Pharmaceutics Research Advisor Tao L. Lowe, Ph.D. Committee Joel Bumgardner, Ph.D. James R. Johnson, Ph.D. Bernd Meibohm, Ph.D. Duane D. Miller, Ph.D. ORCID http://orcid.org/0000-0002-7235-7709 DOI 10.21007/etd.cghs.2017.0429 Comments Two year embargo expires -
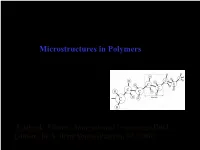
Lecture Notes on Structure and Properties of Engineering Polymers
Structure and Properties of Engineering Polymers Lecture: Microstructures in Polymers Nikolai V. Priezjev Textbook: Plastics: Materials and Processing (Third Edition), by A. Brent Young (Pearson, NJ, 2006). Microstructures in Polymers • Gas, liquid, and solid phases, crystalline vs. amorphous structure, viscosity • Thermal expansion and heat distortion temperature • Glass transition temperature, melting temperature, crystallization • Polymer degradation, aging phenomena • Molecular weight distribution, polydispersity index, degree of polymerization • Effects of molecular weight, dispersity, branching on mechanical properties • Melt index, shape (steric) effects Reading: Chapter 3 of Plastics: Materials and Processing by A. Brent Strong https://www.slideshare.net/NikolaiPriezjev Gas, Liquid and Solid Phases At room temperature Increasing density Solid or liquid? Pitch Drop Experiment Pitch (derivative of tar) at room T feels like solid and can be shattered by a hammer. But, the longest experiment shows that it flows! In 1927, Professor Parnell at UQ heated a sample of pitch and poured it into a glass funnel with a sealed stem. Three years were allowed for the pitch to settle, and in 1930 the sealed stem was cut. From that date on the pitch has slowly dripped out of the funnel, with seven drops falling between 1930 and 1988, at an average of one drop every eight years. However, the eight drop in 2000 and the ninth drop in 2014 both took about 13 years to fall. It turns out to be about 100 billion times more viscous than water! Pitch, before and after being hit with a hammer. http://smp.uq.edu.au/content/pitch-drop-experiment Liquid phases: polymer melt vs. -
![The Effect of Cation Type and H+ on the Catalytic Activity of the Keggin Anion [Pmo12o40]3- in the Oxidative Dehydrogenation Of](https://docslib.b-cdn.net/cover/0912/the-effect-of-cation-type-and-h-on-the-catalytic-activity-of-the-keggin-anion-pmo12o40-3-in-the-oxidative-dehydrogenation-of-1770912.webp)
The Effect of Cation Type and H+ on the Catalytic Activity of the Keggin Anion [Pmo12o40]3- in the Oxidative Dehydrogenation Of
Journal of Catalysis 195, 360–375 (2000) doi:10.1006/jcat.2000.2987, available online at http://www.idealibrary.com on The Effect of Cation Type and H+ on the Catalytic Activity 3 of the Keggin Anion [PMo12O40] in the Oxidative Dehydrogenation of Isobutyraldehyde Ji Hu and Robert C. Burns1 School of Biological and Chemical Sciences, The University of Newcastle, Callaghan 2308, Australia Received March 23, 2000; revised July 4, 2000; accepted July 5, 2000 such as the oxidative dehydrogenation of isobutyric acid The oxidative dehydrogenation of isobutyraldehyde to metha- and the oxidation of methacrolein, both of which yield 3 crolein over [PMo12O40] -containing catalysts has been shown to methacrylic acid (1–5). Methacrylic acid is, in turn, reacted proceed through bulk catalysis-type II, which depends on the rates with methanol to yield methyl methacrylate, an extremely + of diffusion of the redox carriers (H and e ) into the catalyst bulk. important acrylic monomer, which is then polymerized to Variations in catalyst behaviour have been shown to change with give poly(methyl methacrylate). Heteropolyoxometalates the countercation and appear to be related to the polarizing abil- are also active acid catalysts, and processes based both on ity of the cation, which can be represented by the ionic potential their redox and acid–base properties have found commer- (charge/ionic radius). This, in turn, may indicate that the active 3 cial applications (3–5). site at the [PMo12O40] ion is close to an attendant countercation. For the alkali metal ions Li+,Na+,K+,Rb+, and Cs+ as well as the The study of heteropolyoxometalates as oxidation– (isoelectronic) ions of the series Cs+,Ba2+,La3+, and Ce4+, the stud- reduction catalysts has involved primarily Keggin-based 3 ies have shown that conversion generally decreases with increasing structures, principally [PMo12O40] , as well as substi- ionic potential, while selectivity to methacrolein is less affected by tuted species involving replacement of one or more changes in this property. -

Design and Optimization of a Process for the Production of Methyl Methacrylate Via Direct Methylation
processes Article Design and Optimization of a Process for the Production of Methyl Methacrylate via Direct Methylation Taotao Liang 1,2, Xiaogang Guo 2,3,*, Abdulmoseen Segun Giwa 1,4, Jianwei Shi 3, Yujin Li 3, Yan Wei 3, Xiaojuan Wang 5, Xuansong Cao 3, Xiaofeng Tang 3 and Jialun Du 3 1 Green Intelligence Environmental School, Yangtze Normal University, Chongqing 408003, China; [email protected] (T.L.); [email protected] (A.S.G.) 2 Faculty of Materials and Energy, Southwest University, Chongqing 400715, China 3 College of Chemistry and Chemical Engineering, Yangtze Normal University, Chongqing 408003, China; [email protected] (J.S.); [email protected] (Y.L.); [email protected] (Y.W.); [email protected] (X.C.); [email protected] (X.T.); [email protected] (J.D.) 4 State Key Joint Laboratory of Environment Simulation and Pollution Control, School of Environment, Tsinghua University, Beijing 100084, China 5 College of chemistry and bioengineering, Guilin University of Technology, Guilin 541004, China; [email protected] * Correspondence: [email protected]; Tel.: +86-023-72782170 Received: 25 April 2019; Accepted: 6 June 2019; Published: 18 June 2019 Abstract: Methyl methacrylate (MMA) plays a vital role in national productions with broad application. Herein, the production of MMA is realized by the improved eco-friendly direct methylation method using Aspen Plus software. Three novel kinds of energy-saving measures were proposed in this study, including the recycle streams of an aqueous solution, methacrolein (MAL), and methanol, the deployment of double-effect distillation instead of a normal one, and the design of a promising heat-exchange network. -

PATENT OFFICE 2,101,820 PROCESS for the PREPARATION of POLYMERIZABLE UNSATURATED COM POUNDS John C
Patented Dec. 7, 1937 2,101,820 UNITED STATES PATENT OFFICE 2,101,820 PROCESS FOR THE PREPARATION OF POLYMERIZABLE UNSATURATED COM POUNDS John C. Woodhouse, Wilmington, Del, assignor to E. I. du Pont de Nemours & Company, Will mington, Del, a corporation of Delaware No Drawing. Application December 5, 1933, Serial No. 701,053 10 Claims. (Cl. 260-106) This invention relates to a process for the prep included under the term aryl include benzyl, aration of unsaturated polymerizable organic naphthyl, and analogous aryl compounds. It will compounds and more particularly to the prepara be appreciated by those skilled in this art that tion of Such compounds from Saturated organic altho the Substitutions in the X and Y positions 5 compounds containing an isopropyl radical at Will alter the reaction rates and in many in 5 tached to a carbonyl group which likewise carries stances the by-products resulting from the de a hydrogen, hydroxy, aryl, or aralkyl group. hydrogenation, these substitutions are to be given An object of the present invention is to provide but secondary consideration for, generally speak an improved process for the preparation of poly ing, if the compound to be dehydrogenated has the O merizable unsaturated aliphatic and/or aromatic basic formula 10 organic compounds. Another object of the in vention is to provide an improved process for the chi-qi- - O preparation of esters of the unsaturated mono it may be dehydrogenated to an unsaturated poly carboxylic aliphatic organic acids. Another ob merizable compound. ject of the invention is to provide an improved The compounds containing an isobutyryl group, 15 process for the preparation of methacrylic acid. -

Lecture1541230922.Pdf
MODULE-1 Introduction: -The macromolecules are divided between biological and non-biological materials. The biological polymers form the very foundation of life and intelligence, and provide much of the food on which man exists. The non-biological polymers are primarily the synthetic materials used for plastics, fibers and elastomers but a few naturally occurring polymers such as rubber wool and cellulose are included in this class. Today these substances are truly indispensable to mankind because these are essential to his clothing,shelter, transportation, communication as well as the conveniences of modern living. Polymer Biological Non- biological Proteins, starch, wool plastics, fibers easterners Note: Polymer is not said to be as macromolecule, because polymer is composedof repeating units whereas the macromolecules may not be composed of repeating units. Definition: A polymer is a large molecule built up by the repetition of small, simple chemical units known as repeating units which are held together by chemical covalent bonds. These repeating units are called monomer Polymer – ---- poly + meres Many parts In some cases, the repetition is linear but in other cases the chains are branched on interconnected to form three dimensional networks. The repeating units of the polymer are usually equivalent on nearly equivalent to the monomer on the starting material from which the polymer is formed. A higher polymer is one in which the number of repeating units is in excess of about 1000 Degree of polymerization (DP): - The no of repeating units or monomer units in the chain of a polymer is called degree of polymerization (DP) . The molecular weight of an addition polymer is the product of the molecular weight of the repeating unit and the degree of polymerization (DP). -
Experiment 26E LINEAR and CROSSLINKED POLYMERS
Experiment 26E FV-4-13-09 LINEAR AND CROSSLINKED POLYMERS MATERIALS: Resorcinol, 3 M NaOH, formalin, phthalic anhydride, anhydrous sodium acetate, ethylene glycol, glycerol, corn starch, Nylon 6,10 demo, ring stand, Bunsen burner or hot plate, 2 clamps, test tube (20 x 150 mm), 2 test tubes (16 x 125 mm), applicator sticks, weighing boat, polyvinyl alcohol solution, sodium borate solution, stirring rod, 20 mL graduated cylinder, 10 mL graduated cylinder, 2 beakers, 2 plastic beakers, thermometer. PURPOSE: The purpose of this experiment is to study the properties of polymers. LEARNING OBJECTIVES: By the end of this experiment, the student should be able to demonstrate the following proficiencies: 1. Understand the differences between linear and crosslinked polymers. 2. Compare and contrast the properties of polyester fibers. 3. Define the following terms: polymer, monomer, repeat unit, crosslinking, biopolymer. PRE-LAB: Complete the Pre-lab assignment before lab. DISCUSSION: Polymers are extremely large molecules (molar masses from 1000 to greater than 106 g/mole) which result from chemically linking thousands of relatively small molecules called monomers. Dramatic changes in physical properties accompany this process. Monomers, due to their weak intermolecular forces, are either gases, liquids or structurally weak molecular solids. In a polymerization reaction, these are joined together to form larger molecules. As the intermolecular forces between the molecules increase, the mixture becomes more viscous. Eventually the molecules become so large that their chains become entangled. It is this entanglement of the individual polymer chains that gives polymeric materials their characteristic properties. A classic example of the changes accompanying polymerization is given by the conversion of ethylene to polyethylene (PE). -

Methacrylic Acid, CAS No. 79-41-4; March 1996ECETOC (1995A): Joint Assessment of Commodity Chemicals, Methacrylic Acid, CAS No
N port O N N COMMISSIO 837 E INT RESEARCH CENTRE lic acid OH 3 EINECS No: 201-204-4 EUR 19 EUROPEA JO y CH methacr C 2 H CAS No: 79-41-4 European Union Risk Assessment Re Protection 25 : Substances Priority List st European Institute for Health and Consumer 1 Bureau Chemicals Existing Volume European Chemicals Bureau European Union Risk Assessment Report CAS: 79-41-4 EC: 201-204-4 methacrylic acid 25 PL-1 European Union Risk Assessment Report METHACRYLIC ACID CAS No: 79-41-4 EINECS No: 201-204-4 RISK ASSESSMENT LEGAL NOTICE Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of the following information A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa Server (http://europa.eu.int). Cataloguing data can be found at the end of this publication Luxembourg: Office for Official Publications of the European Communities, 2002 ISBN 92-894-1273-9 © European Communities, 2002 Reproduction is authorised provided the source is acknowledged. Printed in Italy METHACRYLIC ACID CAS No: 79-41-4 EINECS No: 201-204-4 RISK ASSESSMENT Final Report, 2002 Germany The risk assessment of methacrylic acid (MAA) has been prepared by Germany on behalf of the European Union. Contact point: Bundesanstalt für Arbeitsschutz und Arbeitsmedizin Anmeldestelle Chemikaliengesetz (BAuA) (Federal Institute for Occupational Safety and Health Notification Unit) Friedrich-Henkel-Weg 1-25 44149 Dortmund Germany Fax: +49 (231) 9071-679 e-mail: [email protected] Date of Last Literature Search: 1995 Review of report by MS Technical Experts finalised: 1999 Final report: 2002 (The last full literature survey was carried out in 1995 - targeted searches (for example on grouting) were carried out subsequently, and information found through scanning certain sources has also been included). -

Methycryolic Acid and Esters, Supp. A
Report No. 11 A METHACRYLIC ACID METHACRYLIC ESTERS Supplement A by DONALD C. THOMAS Contributions by Brij M. Sood and Leslie A. Carmichael May 1974 A private report by the PROCESS ECONOMICS PROGRAM I STANFORD RESEARCH INSTITUTE MENLO PARK, CALIFORNIA I - - CONTENTS 1 INTRODUCTION . 1 2 SUMMARY................,.......... 3 Methyl Methacrylate via Acetone Cyanohydrin . 5 Methyl Methacrylate from Isobutylene via Methacrylonitrile . 6 Methyl Methacrylate from Isobutylene via Methacrylic Acid . 7 3 COMPARISON OF PROCESSES . 9 4 INDUSTRY STATUS . 15 5 METHYL METHACRYLATE VIA ACETONE CYANOHYDRIN ......... 19 Review of Processes ..................... 19 Hydrogen Cyanide Production ................ 19 Acetone Cyanohydrin Production .............. 21 Hydrolysis and Esterification ............... 24 Recovery of Methyl Methacrylate .............. 25 Handling and Disposition of Ammonium Bisulfate ...... 26 Process Description ..................... 29 Hydrogen Cyanide Production ................ 29 Acetone Cyanohydrin Production .............. 32 Hydrolysis and Esterification ............... 33 Purification ....................... 34 Sulfuric Acid Regeneration ................ 34 Process Discussion ..................... 49 Waste Treatment ....................... 50 Cost Estimates ....................... 52 6 METHYL METHACRYLATE FROM ISOBUTYLENE VIA METHACRYLONITRILE ...................... 61 Review of Processes ..................... 61 Methacrylonitrile Production ............... 61 Treatment of Ammoxidation By-Products ........... 65 Hydrolysis and