Sand-Binding Roots in Haemodoraceae: Global Survey and Morphology in a Phylogenetic Context
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cunninghamia Date of Publication: February 2020 a Journal of Plant Ecology for Eastern Australia
Cunninghamia Date of Publication: February 2020 A journal of plant ecology for eastern Australia ISSN 0727- 9620 (print) • ISSN 2200 - 405X (Online) The Australian paintings of Marianne North, 1880–1881: landscapes ‘doomed shortly to disappear’ John Leslie Dowe Australian Tropical Herbarium, James Cook University, Smithfield, Qld 4878 AUSTRALIA. [email protected] Abstract: The 80 paintings of Australian flora, fauna and landscapes by English artist Marianne North (1830-1890), completed during her travels in 1880–1881, provide a record of the Australian environment rarely presented by artists at that time. In the words of her mentor Sir Joseph Dalton Hooker, director of Kew Gardens, North’s objective was to capture landscapes that were ‘doomed shortly to disappear before the axe and the forest fires, the plough and the flock, or the ever advancing settler or colonist’. In addition to her paintings, North wrote books recollecting her travels, in which she presented her observations and explained the relevance of her paintings, within the principles of a ‘Darwinian vision,’ and inevitable and rapid environmental change. By examining her paintings and writings together, North’s works provide a documented narrative of the state of the Australian environment in the late nineteenth- century, filtered through the themes of personal botanical discovery, colonial expansion and British imperialism. Cunninghamia (2020) 20: 001–033 doi: 10.7751/cunninghamia.2020.20.001 Cunninghamia: a journal of plant ecology for eastern Australia © 2020 Royal Botanic Gardens and Domain Trust www.rbgsyd.nsw.gov.au/science/Scientific_publications/cunninghamia 2 Cunninghamia 20: 2020 John Dowe, Australian paintings of Marianne North, 1880–1881 Introduction The Marianne North Gallery in the Royal Botanic Gardens Kew houses 832 oil paintings which Marianne North (b. -
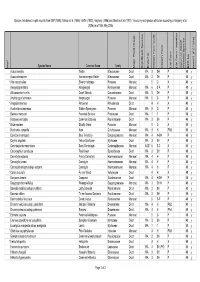
BFS048 Site Species List
Species lists based on plot records from DEP (1996), Gibson et al. (1994), Griffin (1993), Keighery (1996) and Weston et al. (1992). Taxonomy and species attributes according to Keighery et al. (2006) as of 16th May 2005. Species Name Common Name Family Major Plant Group Significant Species Endemic Growth Form Code Growth Form Life Form Life Form - aquatics Common SSCP Wetland Species BFS No kens01 (FCT23a) Wd? Acacia sessilis Wattle Mimosaceae Dicot WA 3 SH P 48 y Acacia stenoptera Narrow-winged Wattle Mimosaceae Dicot WA 3 SH P 48 y * Aira caryophyllea Silvery Hairgrass Poaceae Monocot 5 G A 48 y Alexgeorgea nitens Alexgeorgea Restionaceae Monocot WA 6 S-R P 48 y Allocasuarina humilis Dwarf Sheoak Casuarinaceae Dicot WA 3 SH P 48 y Amphipogon turbinatus Amphipogon Poaceae Monocot WA 5 G P 48 y * Anagallis arvensis Pimpernel Primulaceae Dicot 4 H A 48 y Austrostipa compressa Golden Speargrass Poaceae Monocot WA 5 G P 48 y Banksia menziesii Firewood Banksia Proteaceae Dicot WA 1 T P 48 y Bossiaea eriocarpa Common Bossiaea Papilionaceae Dicot WA 3 SH P 48 y * Briza maxima Blowfly Grass Poaceae Monocot 5 G A 48 y Burchardia congesta Kara Colchicaceae Monocot WA 4 H PAB 48 y Calectasia narragara Blue Tinsel Lily Dasypogonaceae Monocot WA 4 H-SH P 48 y Calytrix angulata Yellow Starflower Myrtaceae Dicot WA 3 SH P 48 y Centrolepis drummondiana Sand Centrolepis Centrolepidaceae Monocot AUST 6 S-C A 48 y Conostephium pendulum Pearlflower Epacridaceae Dicot WA 3 SH P 48 y Conostylis aculeata Prickly Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis juncea Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis setigera subsp. -

Appendix F- H (PDF, 4.64
APPENDIX F Analysis to Investigate TEC SCP20a Presence Perth–Darwin National Highway (Swan Valley Section)– Supplementary Biological Studies 2015 Assessment of the Presence of the TEC SCP20a at Ioppolo Rd, Chittering COFFEY NOVEMBER 2015 TEL. (08) 9315 4688 [email protected] PO Box 50, Applecross WA 6953 www.woodmanenv.com.au Coffey Perth–Darwin National Highway (Swan Valley Section)– Supplementary Biological Studies 2015 Assessment of the presence of the TEC SCP20a at Ioppolo Rd, Chittering Perth–Darwin National Highway (Swan Valley Section) – Supplementary Biological Studies 2015: Assessment of the presence of the TEC SCP20a at Ioppolo Rd, Chittering Prepared for: Coffey Job Number: Coffey15‐28 Report Number: Coffey15‐28‐03 Cover Photograph: Lot M2019 ‐ Ioppolo Road, waypoint 82 (Woodman Environmental) DOCUMENT REVISION AND STATUS Revision Status Originator Internal Internal Client Client Reviewer Review Date Reviewer Review Date A Draft report BL CG/GW 22/10/2015 N. Raymond 16/11/2015 Main Roads B Client Comments BL GW 19/11/2015 D. Morley 26/11/2015 Incorporated Main Roads 0 Final report BL GW 26/11/2015 DISCLAIMER This document is prepared in accordance with and subject to an agreement between Woodman Environmental Consulting Pty Ltd (“Woodman Environmental”) and the client for whom it has been prepared (“Coffey”) and is restricted to those issues that have been raised by the Client in its engagement of Woodman Environmental and prepared eusing th standard of skill and care ordinarily exercised by Environmental Scientists -

Beijing Olympic Mascots
LEVEL – Lower primary FLORAL EMBLEMS DESCRIPTION In these activities, students learn about the floral emblems of Great Britain. They discuss their own responses to the emblems and explore design elements and features including colours, shapes, lines and their purpose before colouring a picture. These cross-curriculum activities contribute to the achievement of the following: Creative and visual arts • Selects, combines and manipulates images, shapes and forms using a range of skills, techniques and processes. English • Interprets and discusses some relationships between ideas, information and events in visual texts for general viewing. SUGGESTED TIME approximately 15-30 minutes for each activity (this may be customised accordingly) WHAT YOU NEED • photographs or actual samples of the floral emblem for your state or territory http://www.anbg.gov.au/emblems/index.html • photographs or actual samples of the floral emblems of Great Britain – Rose (England), Shamrock (Ireland), Thistle (Scotland) and Daffodil (Wales) o http://www.flickr.com/groups/roses/ o http://www.flickr.com/photos/tags/shamrock/clusters/green-irish-stpatricksday/ o http://www.flickr.com/search/?q=Thistle+ o http://www.flickr.com/groups/daffodilworld/ • copies of Student handout • paint, brushes, markers, crayons, glitter and other art materials ACTIVITIES The following activities may be completed independently or combined as part of a more comprehensive learning sequence, lesson or educational program. Please refer to your own state or territory syllabus for more explicit guidelines. Australia’s floral emblems 1. Show the class a picture or sample of Golden Wattle, along with the floral emblem for your state or territory. Ask the class if anyone has these flowers growing in their garden or local area. -

Canberra Bird Notes
canberra ISSN 0314-8211 bird Volume 42 Number 3 December 2017 notes Registered by Australia Post 100001304 CANBERRA ORNITHOLOGISTS GROUP, INC. PO Box 301 Civic Square ACT 2608 2017-18 Committee President Neil Hermes 0413 828 045 Vice-President Vacant Secretary Bill Graham 0466 874 723 Treasurer Vacant 6231 0147 (h) Member Jenny Bounds Member Sue Lashko Member Lia Battisson Member David McDonald Member Paul Fennell Member A.O. (Nick) Nicholls Member Steve Read Email contacts General inquiries [email protected] President [email protected] Canberra Bird Notes [email protected]/[email protected] COG Database Inquiries [email protected] COG Membership [email protected] COG Web Discussion List [email protected] Conservation [email protected] Gang-gang Newsletter [email protected] GBS Coordinator [email protected] Publications for sale [email protected] Unusual bird reports [email protected] Website [email protected] Woodland Project [email protected] Other COG contacts Jenny Bounds Conservation Field Trips Sue Lashko 6251 4485 (h) COG Membership Sandra Henderson 6231 0303 (h) Canberra Bird Notes Editor Michael Lenz 6249 1109 (h) Assistant Editor Kevin Windle 6286 8014 (h) Editor for Annual Bird Report Paul Fennell 6254 1804 (h) Newsletter Editor Sue Lashko, Gail Neumann (SL) 6251 4485 (h) Databases Jaron Bailey 0439 270 835 (a.h.) Garden Bird Survey Duncan McCaskill 6259 1843 (h) Rarities Panel Barbara Allan 6254 6520 (h) Talks Program Organiser Jack Holland 6288 7840 (h) Records Officer Nicki Taws 6251 0303 (h) Website Julian Robinson 6239 6226 (h) Sales Kathy Walter 6241 7639 (h) Waterbird Survey Michael Lenz 6249 1109 (h) Distribution of COG publications Dianne Davey 6254 6324 (h) COG Library Barbara Allan 6254 6520 (h) Please use the General Inquiries email to arrange access to library items or for general enquiries, or contact the Secretary on 0466 874 723. -

Anigozanthos Bicolor Subsp. Minor) Recovery Plan
SMALL TWO-COLOURED KANGAROO PAW (ANIGOZANTHOS BICOLOR SUBSP. MINOR) RECOVERY PLAN Department of Environment and Conservation Kensington Recovery Plan for Anigozanthos bicolor subsp. minor FOREWORD Interim Recovery Plans (IRPs) are developed within the framework laid down in Department of Conservation and Land Management (CALM) [now Department of Environment and Conservation (DEC)] Policy Statements Nos. 44 and 50. Note: the Department of CALM formally became the Department of Environment and Conservation (DEC) in July 2006. DEC will continue to adhere to these Policy Statements until they are revised and reissued. IRPs outline the recovery actions that are required to urgently address those threatening processes most affecting the ongoing survival of threatened taxa or ecological communities, and begin the recovery process. DEC is committed to ensuring that Threatened taxa are conserved through the preparation and implementation of Recovery Plans (RPs) or IRPs, and by ensuring that conservation action commences as soon as possible and, in the case of Critically Endangered (CR) taxa, always within one year of endorsement of that rank by the Minister. This Interim Recovery Plan will operate from May 2006 to April 2011 but will remain in force until withdrawn or replaced. It is intended that, if the taxon is still ranked Critically Endangered (WA), this IRP will be reviewed after five years and the need for further recovery actions assessed. This IRP was given regional approval on 13 February, 2006 and was approved by the Director of Nature Conservation on 22 February, 2006. The allocation of staff time and provision of funds identified in this Interim Recovery Plan is dependent on budgetary and other constraints affecting DEC, as well as the need to address other priorities. -

Pinal AMA Low Water Use/Drought Tolerant Plant List
Arizona Department of Water Resources Pinal Active Management Area Low-Water-Use/Drought-Tolerant Plant List Official Regulatory List for the Pinal Active Management Area Fourth Management Plan Arizona Department of Water Resources 1110 West Washington St. Ste. 310 Phoenix, AZ 85007 www.azwater.gov 602-771-8585 Pinal Active Management Area Low-Water-Use/Drought-Tolerant Plant List Acknowledgements The Pinal Active Management Area (AMA) Low-Water-Use/Drought-Tolerant Plants List is an adoption of the Phoenix AMA Low-Water-Use/Drought-Tolerant Plants List (Phoenix List). The Phoenix List was prepared in 2004 by the Arizona Department of Water Resources (ADWR) in cooperation with the Landscape Technical Advisory Committee of the Arizona Municipal Water Users Association, comprised of experts from the Desert Botanical Garden, the Arizona Department of Transporation and various municipal, nursery and landscape specialists. ADWR extends its gratitude to the following members of the Plant List Advisory Committee for their generous contribution of time and expertise: Rita Jo Anthony, Wild Seed Judy Mielke, Logan Simpson Design John Augustine, Desert Tree Farm Terry Mikel, U of A Cooperative Extension Robyn Baker, City of Scottsdale Jo Miller, City of Glendale Louisa Ballard, ASU Arboritum Ron Moody, Dixileta Gardens Mike Barry, City of Chandler Ed Mulrean, Arid Zone Trees Richard Bond, City of Tempe Kent Newland, City of Phoenix Donna Difrancesco, City of Mesa Steve Priebe, City of Phornix Joe Ewan, Arizona State University Janet Rademacher, Mountain States Nursery Judy Gausman, AZ Landscape Contractors Assn. Rick Templeton, City of Phoenix Glenn Fahringer, Earth Care Cathy Rymer, Town of Gilbert Cheryl Goar, Arizona Nurssery Assn. -

Central Soils Species List
HEAD CENTRAL SOILS SPECIES LIST Start of flowering time: Spring Summer Autumn Winter All Year Common Name Botanical Name Height (m) Flower Colour Flower Time Other Info Trees (Up to 15m) Fraser’s Sheoak Allocasuarina fraseriana 15 brown May-Oct Candle Banksia Banksia attenuata 5-8 yellow Sep-Oct Bull Banksia Banksia grandis 10 yellow Sep-Dec Holly-leaf Banksia Banksia ilicifolia 10 pink & cream Mar-Jan Firewood Banksia °Banksia menziesii 10 pink & red Feb-Aug money water, save Red Flowering Gum Eucalyptus ficifolia 8 red Dec-May WA Coastal Blackbutt Eucalyptus todtiana 9-16 creamy white Feb Coral Gum Eucalyptus torquata 4-11 pink, red Aug-Dec WA Sandplain Woody Pear Xylomelum angustifolium 7 creamy white Dec-Feb WA Shrubs (3 to 5m) Coojong Acacia saligna 5 yellow Aug-Oct garden your back to & bring life Common Woollybush Adenanthos cygnorum 2-4 red Sep-Feb Tree Smokebush Conospermum triplinervium 4.5 greyish white Aug-Nov Red Pokers Hakea bucculenta 4.5 red Aug-Sep WA Royal Hakea Hakea victoria 3 white, colourful foliage Jun-Jul WA Zamia Palm Macrozamia riedlei 3 red cones Sep-Oct River Pea Oxylobium lineare 3 red, yellow Sep-Jan pictured left Shrubs (1 to 3m) Kangaroo Paw Acacia dentifera 3 golden Aug-Nov Prickly Moses Acacia pulchella 1.5 yellow Jun-Oct Basket Flower Adenanthos obovatus 2 scarlet, orange May-Dec manglesii Anigozanthos One-sided Bottlebrush °Calothamnus quadrifidus 1-2 red Aug-Dec Silky-leaved Blood Flower Calothamnus sanguineus 1.5 blood red Mar-Oct Plume Smokebush Conospermum incurvum 0.4-1 white-grey Jul-Nov -

080058-92.02.002.Pdf
'Jeqruq ',{ueq1y 'pusD{u"rd ,Isad sed,{1i(1runuruoc Jeqlo ss sB JoJ pqse^]sq pua {rsrruaq II I I[€^\ 'eprsaeo eq o1 ,(1e41 s4serc! (roloctsp^tp mdQocng) IuJa4 4ru"Jg ol JeuB€raql lsEe-qlnos-ls"e sJapuDerl 11e 'eepJ?I ',{ellBeq^{ sapnlcu! 1 esnecaq uorleSgsa,rur aaJs puB ol qlnos ol lsue'dnuuBN ot Jo Fl.qdBJSoeB erll sB ueJJ8^\ eql esoqc e/l\ lsorrrl€ r{uou anp spuoq l! eJaq,r Je^ry ,([auuoq aqt ol lculstpqns lBcruslog ut"ld Je^rU gocs egl do1 eq1 sso:cr '1sare1m Jo NRo-r{lnos-ls"e luraueSelour Jo eq oslr ueql 'ralry poo.r$lt?tg eq1 uo e8pug repusxelv ol ,{ru 1sq 1ua1dprru qJJBeseJcrlsuou Jo ,l\erleJ aqJ lsua-qlnos-g$os sun-r ifurprmoq prrvJu Jo rueqUou aql 'sn[L '(SS 'd 'uousrrrJoJr[ dn8uJlpl urorJ :OgOt preag) lueuoduoc cglualJs elqs[r8^? lseq luucgruErs € eq ol sesrea JoloJtslaqp e:eql\ eq1 q1m,(4snpw drqcpoo,{ aq1 eEeuuu pnB rolmour ? 'lueue8su"l u.{erp sr,{:epunoq ruaquou eql elq,t\ 'lse/rt pu qlnos IrlSru 11 leqf os I purl pu? uorts^lesuoC 'lseoc sgl ol "es aq1 .{q paprmoq sr lI qmos eqt uo Jo lueulredaq eqt ,{q pelJnpuoJ Bllerlsny {*qlv ot e8pJg alsqumlulq-urrnoe-I eqt uo dn?u1ge1 lsea-rllnos u1 luerua8yuvu lseJoJ Jo lcedur eq1 uo qcJBoser uo.5 u{ 0o€ re^o spuelxe lcFlslpgns uerr"d\ eql Jo ?rterler B ol uorpquluoa v sa ,{luzuud esoP s3^\ IroA\ eqJ qcJReseJamlry ro3 sequoud 'errqsadsred 'EcefoJd luuor8a.r e ur seulluepr puE rIcJBeseJ tuaJrnc ol sJeJeJ ur"{ ol pernJuoo queuodwoc esoql go 'BroU '(9961 lseloJ lueuecqd r"lncsBl u,llou{ eql 3o 1s1 e quaserd 11 s8q lculslPgns uerrB eloq^1 eql JoJ P€lqBua \ p-rceg rnras) lclrtspgns lBcruslog -

A Potential New Cutflower for Australia - Haemodorum Coccineum
A Potential New Cutflower For Australia - Haemodorum Coccineum A report for the Rural Industries Research and Development Corporation by Margaret Johnston and Alenna McMah September 2006 RIRDC Publication No 06/087 RIRDC Project No UQ-117A © 2006 Rural Industries Research and Development Corporation. All rights reserved. ISBN 1 74151 350 2 ISSN 1440-6845 Haemodorum coccineum production in south-east Queensland Publication No. 06/087 Project No. UQ117A The information contained in this publication is intended for general use to assist public knowledge and discussion and to help improve the development of sustainable industries. The information should not be relied upon for the purpose of a particular matter. Specialist and/or appropriate legal advice should be obtained before any action or decision is taken on the basis of any material in this document. The Commonwealth of Australia, Rural Industries Research and Development Corporation, the authors or contributors do not assume liability of any kind whatsoever resulting from any person's use or reliance upon the content of this document. This publication is copyright. However, RIRDC encourages wide dissemination of its research, providing the Corporation is clearly acknowledged. For any other enquiries concerning reproduction, contact the Publications Manager on phone 02 6272 3186. Researcher Contact Details Dr Margaret Johnston Mrs Alenna McMah Centre for Native Floriculture Boomajarril Native Flower Farm Phone: 07 54601240 Phone: 07 54665668 Fax: 07 54601112 Fax: 07 54665668 Email: [email protected] Email: [email protected] In submitting this report, the researcher has agreed to RIRDC publishing this material in its edited form. RIRDC Contact Details Rural Industries Research and Development Corporation Level 2, 15 National Circuit BARTON ACT 2600 PO Box 4776 KINGSTON ACT 2604 Phone: 02 6272 4819 Fax: 02 6272 5877 Email: [email protected]. -

Jandakot Airport Dieback Management Plan Conservation Management
JANDAKOT AIRPORT DIEBACK MANAGEMENT PLAN CONSERVATION MANAGEMENT PLAN APPENDIX C Jandakot Airport Holdings Pty Ltd 16 Eagle Drive Jandakot WA 6164 Ref: Cmp Appendix C Dieback Management Plan V9 Page 1 Version 9 Saved on June 26, 2015 Saved At: Q:\Controlled Documents\Manuals\Conservation Management Plan\CMP Appendix C Dieback Management Plan V9.doc TABLE OF CONTENTS 1 BACKGROUND ........................................................................................................... 3 2 MANAGEMENT ........................................................................................................ 4 2.1 Dieback Treatment ................................................................................................ 4 2.1.1 Phosphite Application ........................................................................................ 4 2.2 Dieback Prevention and Containment .................................................................... 4 2.2.1 Access ............................................................................................................... 4 2.2.2 Construction/Earthmoving .................................................................................. 5 2.2.3 Drainage ............................................................................................................ 5 2.2.4 Landscaping and Revegetation .......................................................................... 6 2.2.5 Bushfire Management ........................................................................................ 7 -

WRA Species Report
Family: Haemodoraceae Taxon: Anigozanthos flavidus Synonym: NA Common Name: Evergreen kangaroo paw Tall kangaroo paw Questionaire : current 20090513 Assessor: Chuck Chimera Designation: H(HPWRA) Status: Assessor Approved Data Entry Person: Chuck Chimera WRA Score 12 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- Intermediate substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 n 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 n 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 y 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see y Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see y Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see Appendix 2) 401 Produces spines, thorns or burrs y=1, n=0 n 402 Allelopathic y=1, n=0 403 Parasitic y=1, n=0 n 404 Unpalatable to grazing animals y=1, n=-1 405 Toxic to animals y=1, n=0 n 406 Host for