Weighted Burden Analysis of Exome-Sequenced Late
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Redefining the Specificity of Phosphoinositide-Binding by Human
bioRxiv preprint doi: https://doi.org/10.1101/2020.06.20.163253; this version posted June 21, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Redefining the specificity of phosphoinositide-binding by human PH domain-containing proteins Nilmani Singh1†, Adriana Reyes-Ordoñez1†, Michael A. Compagnone1, Jesus F. Moreno Castillo1, Benjamin J. Leslie2, Taekjip Ha2,3,4,5, Jie Chen1* 1Department of Cell & Developmental Biology, University of Illinois at Urbana-Champaign, Urbana, IL 61801; 2Department of Biophysics and Biophysical Chemistry, Johns Hopkins University School of Medicine, Baltimore, MD 21205; 3Department of Biophysics, Johns Hopkins University, Baltimore, MD 21218; 4Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD 21205; 5Howard Hughes Medical Institute, Baltimore, MD 21205, USA †These authors contributed equally to this work. *Correspondence: [email protected]. bioRxiv preprint doi: https://doi.org/10.1101/2020.06.20.163253; this version posted June 21, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. ABSTRACT Pleckstrin homology (PH) domains are presumed to bind phosphoinositides (PIPs), but specific interaction with and regulation by PIPs for most PH domain-containing proteins are unclear. Here we employed a single-molecule pulldown assay to study interactions of lipid vesicles with full-length proteins in mammalian whole cell lysates. -

Targeting PH Domain Proteins for Cancer Therapy
The Texas Medical Center Library DigitalCommons@TMC The University of Texas MD Anderson Cancer Center UTHealth Graduate School of The University of Texas MD Anderson Cancer Biomedical Sciences Dissertations and Theses Center UTHealth Graduate School of (Open Access) Biomedical Sciences 12-2018 Targeting PH domain proteins for cancer therapy Zhi Tan Follow this and additional works at: https://digitalcommons.library.tmc.edu/utgsbs_dissertations Part of the Bioinformatics Commons, Medicinal Chemistry and Pharmaceutics Commons, Neoplasms Commons, and the Pharmacology Commons Recommended Citation Tan, Zhi, "Targeting PH domain proteins for cancer therapy" (2018). The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access). 910. https://digitalcommons.library.tmc.edu/utgsbs_dissertations/910 This Dissertation (PhD) is brought to you for free and open access by the The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences at DigitalCommons@TMC. It has been accepted for inclusion in The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access) by an authorized administrator of DigitalCommons@TMC. For more information, please contact [email protected]. TARGETING PH DOMAIN PROTEINS FOR CANCER THERAPY by Zhi Tan Approval page APPROVED: _____________________________________________ Advisory Professor, Shuxing Zhang, Ph.D. _____________________________________________ -

Supporting Information
Supporting Information Pouryahya et al. SI Text Table S1 presents genes with the highest absolute value of Ricci curvature. We expect these genes to have significant contribution to the network’s robustness. Notably, the top two genes are TP53 (tumor protein 53) and YWHAG gene. TP53, also known as p53, it is a well known tumor suppressor gene known as the "guardian of the genome“ given the essential role it plays in genetic stability and prevention of cancer formation (1, 2). Mutations in this gene play a role in all stages of malignant transformation including tumor initiation, promotion, aggressiveness, and metastasis (3). Mutations of this gene are present in more than 50% of human cancers, making it the most common genetic event in human cancer (4, 5). Namely, p53 mutations play roles in leukemia, breast cancer, CNS cancers, and lung cancers, among many others (6–9). The YWHAG gene encodes the 14-3-3 protein gamma, a member of the 14-3-3 family proteins which are involved in many biological processes including signal transduction regulation, cell cycle pro- gression, apoptosis, cell adhesion and migration (10, 11). Notably, increased expression of 14-3-3 family proteins, including protein gamma, have been observed in a number of human cancers including lung and colorectal cancers, among others, suggesting a potential role as tumor oncogenes (12, 13). Furthermore, there is evidence that loss Fig. S1. The histogram of scalar Ricci curvature of 8240 genes. Most of the genes have negative scalar Ricci curvature (75%). TP53 and YWHAG have notably low of p53 function may result in upregulation of 14-3-3γ in lung cancer Ricci curvatures. -

Mouse Osbpl8 Conditional Knockout Project (CRISPR/Cas9)
https://www.alphaknockout.com Mouse Osbpl8 Conditional Knockout Project (CRISPR/Cas9) Objective: To create a Osbpl8 conditional knockout Mouse model (C57BL/6J) by CRISPR/Cas-mediated genome engineering. Strategy summary: The Osbpl8 gene (NCBI Reference Sequence: NM_175489 ; Ensembl: ENSMUSG00000020189 ) is located on Mouse chromosome 10. 24 exons are identified, with the ATG start codon in exon 2 and the TAG stop codon in exon 24 (Transcript: ENSMUST00000105275). Exon 5 will be selected as conditional knockout region (cKO region). Deletion of this region should result in the loss of function of the Mouse Osbpl8 gene. To engineer the targeting vector, homologous arms and cKO region will be generated by PCR using BAC clone RP23-456P17 as template. Cas9, gRNA and targeting vector will be co-injected into fertilized eggs for cKO Mouse production. The pups will be genotyped by PCR followed by sequencing analysis. Note: Mice homozygous for a gene trap allele exhibit elevated of HDL and gender-specific alterations in lipid metabolism. Exon 5 starts from about 8.17% of the coding region. The knockout of Exon 5 will result in frameshift of the gene. The size of intron 4 for 5'-loxP site insertion: 21997 bp, and the size of intron 5 for 3'-loxP site insertion: 7421 bp. The size of effective cKO region: ~571 bp. The cKO region does not have any other known gene. Page 1 of 7 https://www.alphaknockout.com Overview of the Targeting Strategy Wildtype allele gRNA region 5' gRNA region 3' 1 5 24 Targeting vector Targeted allele Constitutive KO allele (After Cre recombination) Legends Exon of mouse Osbpl8 Homology arm cKO region loxP site Page 2 of 7 https://www.alphaknockout.com Overview of the Dot Plot Window size: 10 bp Forward Reverse Complement Sequence 12 Note: The sequence of homologous arms and cKO region is aligned with itself to determine if there are tandem repeats. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0304845 A1 Loboda Et Al
US 201403.04845A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0304845 A1 Loboda et al. (43) Pub. Date: Oct. 9, 2014 54) ALZHEMIERS DISEASE SIGNATURE Publicationublication ClassificatiClassification MARKERS AND METHODS OF USE (51) Int. Cl. (71) Applicant: MERCKSHARP & DOHME CORP, CI2O I/68 (2006.01) Rahway, NJ (US) AOIK 67/027 (2006.01) (52) U.S. Cl. (72) Inventors: SIES yet.sS); CPC .......... CI2O I/6883 (2013.01); A0IK 67/0275 Icnaei NepoZnyn, Yeadon, (2013.01) le.East,Italia; David J. Stone, Wyncote, USPC .............. 800/12:536/23.5:536/23.2:506/17 (US); Keith Tanis, Quakertown, PA (US); William J. Ray, Juniper, FL (US) (57) ABSTRACT (21) Appl. No.: 14/354,622 Methods, biomarkers, and expression signatures are dis 1-1. closed for assessing the disease progression of Alzheimer's (22) PCT Filed: Oct. 26, 2012 disease (AD). In one embodiment, BioAge (biological age), NdStress (neurodegenerative stress), Alz (Alzheimer), and (86). PCT No.: PCT/US12A62218 Inflame (inflammation) are used as biomarkers of AD pro S371 (c)(1), gression. In another aspect, the invention comprises a gene (2), (4) Date: Apr. 28, 2014 signature for evaluating disease progression. In still another Related U.S. Application Data abiliticises in (60) Provisional application No. 61/553,400, filed on Oct. used to identify animal models for use in the development and 31, 2011. evaluation of therapeutics for the treatment of AD. Patent Application Publication Oct. 9, 2014 Sheet 1 of 16 US 2014/0304845 A1 Y : : O O O O O O O O D O v v CN On cy Patent Application Publication Oct. -

Transcriptome Alterations of Vascular Smooth Muscle Cells in Aortic Wall of Myocardial Infarction Patients
This document is downloaded from DR‑NTU (https://dr.ntu.edu.sg) Nanyang Technological University, Singapore. Transcriptome alterations of vascular smooth muscle cells in aortic wall of myocardial infarction patients Wongsurawat, Thidathip; Woo, Chin Cheng; Giannakakis, Antonis; Lin, Xiao Yun; Cheow, Esther Sok Hwee; Lee, Chuen Neng; Richards, Mark; Sze, Siu Kwan; Nookaew, Intawat; Sorokin, Vitaly; Kuznetsov, Vladimir Andreevich 2018 Wongsurawat, T., Woo, C. C., Giannakakis, A., Lin, X. Y., Cheow, E. S. H., Lee, C. N., et al. (2018). Transcriptome alterations of vascular smooth muscle cells in aortic wall of myocardial infarction patients. Data in Brief, 17, 1112‑1135. https://hdl.handle.net/10356/85590 https://doi.org/10.1016/j.dib.2018.01.108 © 2018 The Authors. Published by Elsevier Inc. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/). Downloaded on 09 Oct 2021 06:21:01 SGT Data in Brief 17 (2018) 1112–1135 Contents lists available at ScienceDirect Data in Brief journal homepage: www.elsevier.com/locate/dib Data Article Transcriptome alterations of vascular smooth muscle cells in aortic wall of myocardial infarction patients Thidathip Wongsurawat a,b, Chin Cheng Woo c, Antonis Giannakakis a, Xiao Yun Lin d, Esther Sok Hwee Cheow e, Chuen Neng Lee c,d, Mark Richards f,g, Siu Kwan Sze e, Intawat Nookaew b, Vladimir A. Kuznetsov a,h, Vitaly Sorokin c,d,⁎ a Department of Genome and Gene Expression Data Analysis, Bioinformatics Institute, Agency for Science, Technology and Research (A*STAR), -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in -

Pancreatic Intraductal Tubulopapillary Neoplasm Is Genetically Distinct from Intraductal Papillary Mucinous Neoplasm and Ductal Adenocarcinoma
Modern Pathology (2017) 30, 1760–1772 1760 © 2017 USCAP, Inc All rights reserved 0893-3952/17 $32.00 Pancreatic intraductal tubulopapillary neoplasm is genetically distinct from intraductal papillary mucinous neoplasm and ductal adenocarcinoma Olca Basturk1, Michael F Berger1, Hiroshi Yamaguchi2, Volkan Adsay3, Gokce Askan1, Umesh K Bhanot1, Ahmet Zehir1, Fatima Carneiro4, Seung-Mo Hong5, Giuseppe Zamboni6, Esra Dikoglu7, Vaidehi Jobanputra7,8, Kazimierz O Wrzeszczynski7, Serdar Balci3, Peter Allen9, Naoki Ikari10, Shoko Takeuchi10, Hiroyuki Akagawa10, Atsushi Kanno11, Tooru Shimosegawa11, Takanori Morikawa12, Fuyuhiko Motoi12, Michiaki Unno12, Ryota Higuchi13, Masakazu Yamamoto13, Kyoko Shimizu14, Toru Furukawa15 and David S Klimstra1 1Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY, USA; 2Department of Pathology, Tokyo Medical University, Tokyo, Japan; 3Department of Pathology, Emory University, Atlanta, GA, USA; 4Department of Pathology, Centro Hospitalar São João/Faculty of Medicine of Porto University and Institute for Research and Innovation in Health/Institute of Molecular Pathology and Immunology of the University of Porto (Ipatimup), Porto, Portugal; 5Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea; 6Department of Pathology, University of Verona, Ospedale S.C.-Don Calabria-Negrar, Verona, Italy; 7New York Genome Center, Molecular Diagnostics, New York, NY, USA; 8Department of Pathology, Colombia University Medical Center, New York, NY, USA; 9Department -

Nº Ref Uniprot Proteína Péptidos Identificados Por MS/MS 1 P01024
Document downloaded from http://www.elsevier.es, day 26/09/2021. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. Nº Ref Uniprot Proteína Péptidos identificados 1 P01024 CO3_HUMAN Complement C3 OS=Homo sapiens GN=C3 PE=1 SV=2 por 162MS/MS 2 P02751 FINC_HUMAN Fibronectin OS=Homo sapiens GN=FN1 PE=1 SV=4 131 3 P01023 A2MG_HUMAN Alpha-2-macroglobulin OS=Homo sapiens GN=A2M PE=1 SV=3 128 4 P0C0L4 CO4A_HUMAN Complement C4-A OS=Homo sapiens GN=C4A PE=1 SV=1 95 5 P04275 VWF_HUMAN von Willebrand factor OS=Homo sapiens GN=VWF PE=1 SV=4 81 6 P02675 FIBB_HUMAN Fibrinogen beta chain OS=Homo sapiens GN=FGB PE=1 SV=2 78 7 P01031 CO5_HUMAN Complement C5 OS=Homo sapiens GN=C5 PE=1 SV=4 66 8 P02768 ALBU_HUMAN Serum albumin OS=Homo sapiens GN=ALB PE=1 SV=2 66 9 P00450 CERU_HUMAN Ceruloplasmin OS=Homo sapiens GN=CP PE=1 SV=1 64 10 P02671 FIBA_HUMAN Fibrinogen alpha chain OS=Homo sapiens GN=FGA PE=1 SV=2 58 11 P08603 CFAH_HUMAN Complement factor H OS=Homo sapiens GN=CFH PE=1 SV=4 56 12 P02787 TRFE_HUMAN Serotransferrin OS=Homo sapiens GN=TF PE=1 SV=3 54 13 P00747 PLMN_HUMAN Plasminogen OS=Homo sapiens GN=PLG PE=1 SV=2 48 14 P02679 FIBG_HUMAN Fibrinogen gamma chain OS=Homo sapiens GN=FGG PE=1 SV=3 47 15 P01871 IGHM_HUMAN Ig mu chain C region OS=Homo sapiens GN=IGHM PE=1 SV=3 41 16 P04003 C4BPA_HUMAN C4b-binding protein alpha chain OS=Homo sapiens GN=C4BPA PE=1 SV=2 37 17 Q9Y6R7 FCGBP_HUMAN IgGFc-binding protein OS=Homo sapiens GN=FCGBP PE=1 SV=3 30 18 O43866 CD5L_HUMAN CD5 antigen-like OS=Homo -

Viral Driven Epigenetic Events Alter the Expression of Cancer-Related
www.nature.com/scientificreports OPEN Viral driven epigenetic events alter the expression of cancer- related genes in Epstein-Barr- Received: 6 March 2017 Accepted: 7 June 2017 virus naturally infected Burkitt Published: xx xx xxxx lymphoma cell lines Hector Hernandez-Vargas1, Henri Gruffat2,3,4,5,6, Marie Pierre Cros1, Audrey Diederichs1, Cécilia Sirand1, Romina C. Vargas-Ayala1, Antonin Jay1, GeoffroyD urand1, Florence Le Calvez-Kelm1, Zdenko Herceg1, Evelyne Manet2,3,4,5,6, Christopher P. Wild1, Massimo Tommasino1 & Rosita Accardi1 Epstein-Barr virus (EBV) was identified as the first human virus to be associated with a human malignancy, Burkitt’s lymphoma (BL), a pediatric cancer endemic in sub-Saharan Africa. The exact mechanism of how EBV contributes to the process of lymphomagenesis is not fully understood. Recent studies have highlighted a genetic difference between endemic (EBV+) and sporadic (EBV−) BL, with the endemic variant showing a lower somatic mutation load, which suggests the involvement of an alternative virally-driven process of transformation in the pathogenesis of endemic BL. We tested the hypothesis that a global change in DNA methylation may be induced by infection with EBV, possibly thereby accounting for the lower mutation load observed in endemic BL. Our comparative analysis of the methylation profiles of a panel of BL derived cell lines, naturally infected or not with EBV, revealed that the presence of the virus is associated with a specific pattern of DNA methylation resulting in altered expression of cellular genes with a known or potential role in lymphomagenesis. These included ID3, a gene often found to be mutated in sporadic BL. -
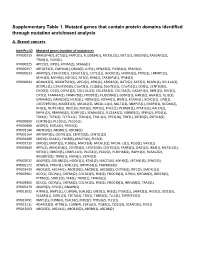
Supplementary Table 1. Mutated Genes That Contain Protein Domains Identified Through Mutation Enrichment Analysis
Supplementary Table 1. Mutated genes that contain protein domains identified through mutation enrichment analysis A. Breast cancers InterPro ID Mutated genes (number of mutations) IPR000219 ARHGEF4(2), ECT2(1), FARP1(1), FLJ20184(1), MCF2L2(1), NET1(1), OBSCN(5), RASGRF2(2), TRAD(1), VAV3(1) IPR000225 APC2(2), JUP(1), KPNA5(2), SPAG6(1) IPR000357 ARFGEF2(2), CMYA4(1), DRIM(2), JUP(1), KPNA5(2), PIK3R4(1), SPAG6(1) IPR000533 AKAP9(2), C10orf39(1), C20orf23(1), CUTL1(1), HOOK1(1), HOOK3(1), KTN1(2), LRRFIP1(3), MYH1(3), MYH9(2), NEF3(1), NF2(1), RSN(1), TAX1BP1(1), TPM4(1) IPR000694 ADAM12(3), ADAMTS19(1), APC2(2), APXL(1), ARID1B(1), BAT2(2), BAT3(1), BCAR1(1), BCL11A(2), BCORL1(1), C14orf155(3), C1orf2(1), C1QB(1), C6orf31(1), C7orf11(1), CD2(1), CENTD3(3), CHD5(3), CIC(3), CMYA1(2), COL11A1(3), COL19A1(2), COL7A1(3), DAZAP1(1), DBN1(3), DVL3(1), EIF5(1), FAM44A(1), FAM47B(1), FHOD1(1), FLJ20584(1), G3BP2(2), GAB1(2), GGA3(1), GLI1(3), GPNMB(2), GRIN2D(3), HCN3(1), HOXA3(2), HOXA4(1), IRS4(1), KCNA5(1), KCNC2(1), LIP8(1), LOC374955(1), MAGEE1(2), MICAL1(2), MICAL‐L1(1), MLLT2(1), MMP15(1), N4BP2(1), NCOA6(2), NHS(1), NUP214(3), ODZ1(3), PER1(2), PER2(1), PHC1(1), PLXNB1(1), PPM1E(2), RAI17(2), RAPH1(2), RBAF600(2), SCARF2(1), SEMA4G(1), SLC16A2(1), SORBS1(1), SPEN(2), SPG4(1), TBX1(1), TCF1(2), TCF7L1(1), TESK1(1), THG‐1(1), TP53(18), TRIF(1), ZBTB3(2), ZNF318(2) IPR000909 CENTB1(2), PLCB1(1), PLCG1(1) IPR000998 AEGP(3), EGFL6(2), PRSS7(1) IPR001140 ABCB10(2), ABCB6(1), ABCB8(2) IPR001164 ARFGAP3(1), CENTB1(2), CENTD3(3), CENTG1(2) IPR001589