Primary and Secondary Consequences of Rotator Cuff
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rotator Cuff and Subacromial Impingement Syndrome: Anatomy, Etiology, Screening, and Treatment
Rotator Cuff and Subacromial Impingement Syndrome: Anatomy, Etiology, Screening, and Treatment The glenohumeral joint is the most mobile joint in the human body, but this same characteristic also makes it the least stable joint.1-3 The rotator cuff is a group of muscles that are important in supporting the glenohumeral joint, essential in almost every type of shoulder movement.4 These muscles maintain dynamic joint stability which not only avoids mechanical obstruction but also increases the functional range of motion at the joint.1,2 However, dysfunction of these stabilizers often leads to a complex pattern of degeneration, rotator cuff tear arthropathy that often involves subacromial impingement.2,22 Rotator cuff tear arthropathy is strikingly prevalent and is the most common cause of shoulder pain and dysfunction.3,4 It appears to be age-dependent, affecting 9.7% of patients aged 20 years and younger and increasing to 62% of patients of 80 years and older ( P < .001); odds ratio, 15; 95% CI, 9.6-24; P < .001.4 Etiology for rotator cuff pathology varies but rotator cuff tears and tendinopathy are most common in athletes and the elderly.12 It can be the result of a traumatic event or activity-based deterioration such as from excessive use of arms overhead, but some argue that deterioration of these stabilizers is part of the natural aging process given the trend of increased deterioration even in individuals who do not regularly perform overhead activities.2,4 The factors affecting the rotator cuff and subsequent treatment are wide-ranging. The major objectives of this exposition are to describe rotator cuff anatomy, biomechanics, and subacromial impingement; expound upon diagnosis and assessment; and discuss surgical and conservative interventions. -

Musculoskeletal Diagnostic Imaging
Musculoskeletal Diagnostic Imaging Vivek Kalia, MD MPH October 02, 2019 Course: Sports Medicine for the Primary Care Physician Department of Radiology University of Michigan @VivekKaliaMD [email protected] Objectives • To review anatomy of joints which commonly present for evaluation in the primary care setting • To review basic clinical features of particular musculoskeletal conditions affecting these joints • To review key imaging features of particular musculoskeletal conditions affecting these joints Outline • Joints – Shoulder – Hip • Rotator Cuff Tendinosis / • Osteoarthritis Tendinitis • (Greater) Trochanteric bursitis • Rotator Cuff Tears • Hip Abductor (Gluteal Tendon) • Adhesive Capsulitis (Frozen Tears Shoulder) • Hamstrings Tendinosis / Tears – Elbow – Knee • Lateral Epicondylitis • Osteoarthritis • Medical Epicondylitis • Popliteal / Baker’s cyst – Hand/Wrist • Meniscus Tear • Rheumatoid Arthritis • Ligament Tear • Osteoarthritis • Cartilage Wear Outline • Joints – Ankle/Foot • Osteoarthritis • Plantar Fasciitis • Spine – Degenerative Disc Disease – Wedge Compression Deformity / Fracture Shoulder Shoulder Rotator Cuff Tendinosis / Tendinitis • Rotator cuff comprised of 4 muscles/tendons: – Supraspinatus – Infraspinatus – Teres minor – Subscapularis • Theory of rotator cuff degeneration / tearing with time: – Degenerative partial-thickness tears allow superior migration of the humeral head in turn causes abrasion of the rotator cuff tendons against the undersurface of the acromion full-thickness tears may progress to -

Impingement Syndrome and Tears of the Rotator Cuff
Impingement Syndrome and Tears of the Rotator Cuff Dr Keith Holt Impingement is a very common problem in which the tendons of the rotator cuff (predominantly supraspinatus) rub on the underside of the acromion (the bone at the point of the shoulder). This causes pain due to the repeated rubbing of those tendons and it is especially bad with certain positions of the arm. In particular it is difficult to put the arm behind the back and to use it in the elevated position. This makes it difficult to drive, change gears, hang clothes, comb one’s hair, and even to lie on the affected shoulder. The cause of this problem can be: How does the shoulder work? The shoulder, like the hip, is a ball and socket joint (like a tow 1) A muscle imbalance problem due to poor functioning bar). Unlike the hip however, the socket is very small and is of the rotator cuff tendons themselves; thus allowing the not big enough to hold the head of the humerus (the ball) arm to ride up and rub on the acromion, squashing the in place. This gives the joint a large range of motion but, as rotator cuff tendons in the process: or a consequence, it also means that it is potentially unstable. 2) A mechanical problem where the space for the tendon To function normally, muscles on both sides of the joint must is inadequate. One way this can occur is with an injury work together to hold the joint in place during movement. to the tendon itself which causes swelling of that tendon This means that when the deltoid muscle (see diagrams) lifts such that it becomes too large for the space at hand the arm out from the side of the body, the supraspinatus and [primary tendonitis (inflammation of the tendon) with other muscles of the rotator cuff must pull down on the top secondary impingement [rubbing of the tendon on the of the humerus. -
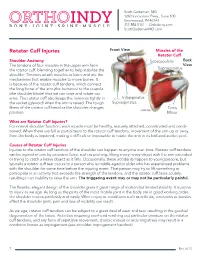
Rotator Cuff Injuries
Scott Gudeman, MD 1260 Innovation Pkwy., Suite 100 Greenwood, IN 46143 317.884.5161 OrthoIndy.com ScottGudemanMD.com Rotator Cuff Injuries Front View Muscles of the Rotator Cuff Shoulder Anatomy Subscapularis Back The tendons of four muscles in the upper arm form View Supraspinatus the rotator cuff, blending together to help stabilize the shoulder. Tendons attach muscles to bone and are the mechanisms that enable muscles to move bones. It is because of the rotator cuff tendons, which connect the long bone of the arm (the humerus) to the scapula (the shoulder blade) that we can raise and rotate our arms. The rotator cuff also keeps the humerus tightly in Infraspinatus the socket (glenoid) when the arm is raised. The tough Supraspinatus fibers of the rotator cuff bend as the shoulder changes Teres position. Minor What are Rotator Cuff Injuries? For normal shoulder function, each muscle must be healthy, securely attached, coordinated and condi- tioned. When there are full or partial tears to the rotator cuff tendons, movement of the arm up or away from the body is impaired, making it difficult or impossible to rotate the arm in its ball-and-socket joint. Causes of Rotator Cuff Injuries Injuries to the rotator cuff tendons of the shoulder can happen to anyone over time. Rotator cuff tendons can be injured or torn by excessive force, such as pitching, lifting a very heavy object with the arm extended or trying to catch a heavy object as it falls. Occasionally, these accidents happen to young people, but typically a rotator cuff tear occurs to a person who is middle-aged or older who has experienced problems with the shoulder for some time before the injuring event. -

Rotator Cuff Tendinitis Shoulder Joint Replacement Mallet Finger Low
We would like to thank you for choosing Campbell Clinic to care for you or your family member during this time. We believe that one of the best ways to ensure quality care and minimize reoccurrences is through educating our patients on their injuries or diseases. Based on the information obtained from today's visit and the course of treatment your physician has discussed with you, the following educational materials are recommended for additional information: Shoulder, Arm, & Elbow Hand & Wrist Spine & Neck Fractures Tears & Injuries Fractures Diseases & Syndromes Fractures & Other Injuries Diseases & Syndromes Adult Forearm Biceps Tear Distal Radius Carpal Tunnel Syndrome Cervical Fracture Chordoma Children Forearm Rotator Cuff Tear Finger Compartment Syndrome Thoracic & Lumbar Spine Lumbar Spine Stenosis Clavicle Shoulder Joint Tear Hand Arthritis of Hand Osteoporosis & Spinal Fx Congenital Scoliosis Distal Humerus Burners & Stingers Scaphoid Fx of Wrist Dupuytren's Comtracture Spondylolysis Congenital Torticollis Shoulder Blade Elbow Dislocation Thumb Arthritis of Wrist Spondylolisthesis Kyphosis of the Spine Adult Elbow Erb's Palsy Sprains, Strains & Other Injuries Kienböck's Disease Lumbar Disk Herniation Scoliosis Children Elbow Shoulder Dislocation Sprained Thumb Ganglion Cyst of the Wrist Neck Sprain Scoliosis in Children Diseases & Syndromes Surgical Treatments Wrist Sprains Arthritis of Thumb Herniated Disk Pack Pain in Children Compartment Syndrome Total Shoulder Replacement Fingertip Injuries Boutonnière Deformity Treatment -
Rotator Cuff Bursitis
Jonathan M. Cooper, D.O. 2700 Vikings Circle Jessica Cicero, PA-C Eagan MN 55121 952-456-7617 Rotator Cuff Bursitis What is a bursa? A bursa is a fluid filled sac that sits between tissue and a bone. They help to reduce friction as the tissues glide across the bone. In the shoulder, the subacromial bursa sits between the acromion and the rotator cuff. The rotator cuff is a group of muscles and tendons that helps to lift and rotate the shoulder. Why do they become irritated? The rotator cuff bursa can become irritated for a variety of reasons. Excessive use of the shoulder can sometime cause inflammation and swelling within the bursa. This can occur from repetitive activity, improper posture or mechanics, or a traumatic event. Some people may be more prone to this condition due to the shape of their bones. What are the symptoms? The symptoms of rotator cuff bursitis can be very similar to a rotator cuff tear. Typically, patients complain of pain along the side or back of the shoulder, especially with overhead activity. Often times, the pain is worse with any lifting away from the body. Occasionally, people describe pain at night or difficulty sleeping. Do I need surgery? Typically, people with rotator cuff bursitis can be treated with conservative treatment including modifying activities, over-the-counter anti-inflammatories, physical therapy, and/or injections. The major focus of treatment involves physical therapy to work on stretching, muscular balance, and proper posture. If modifications in your activity and appropriate physical therapy are not effective in reducing symptoms, then corticosteroid injections may be considered. -

Standard of Care: Shoulder Impingement ICD 9 Codes
BRIGHAM AND WOMEN’S HOSPITAL Department of Rehabilitation Services Physical Therapy Standard of Care: Shoulder Impingement ICD 9 Codes: 726.10 - Rotator cuff syndrome of shoulder and allied disorders 840 - Sprains and strains of shoulder and upper arm Case Type / Diagnosis: Shoulder impingement. Indications for Treatment: Subacromial impingement with rotator cuff tendinopathy is a very common condition seen by both orthopedic surgeons and physical therapists. 23, 4, 18 Impingement syndrome as defined by Neer refers to a pathological condition in which the suprahumeral structures are compressed against the anteroinferior aspect of the acromion and/or the coracoacromial ligament. 23, 4, 17, 34 Impingement can also occur internally between the humerus, glenoid rim, and labrum. 9 The structures most often involved are the rotator cuff tendons, the long head of the biceps and the subacromial bursa.4 Rotator cuff syndrome is a term that is often used to describe the process when both rotator cuff tendinitis and impingement are thought to be occurring simultaneously. 23 There are four major causes of rotator cuff tendinopathy: external impingement, internal anatomical impingement, functional overload and intrinsic tendinopathy. 23 Rotator cuff tears can occur either traumatically or by the result of the degenerative process of tendinosis. Rotator cuff tears (RCT) are frequent and increase with age, yet the varying functional implications of a tear can have a unique and dramatic impact on a patient’s daily life. The presence of a RCT can cause a vast array of impairments and associated dysfunctions. This can be the result of many variables including: age of the individual, activity level of an individual, size of the tear, location of tear, number of tendons involved, and overall rotator cuff tissue quality, as well as the presence or absence of other pathology within the shoulder complex. -

Shoulder Tendonitis
Shoulder Tendonitis Brett Sanders, MD Center For Sports Medicine and Orthopaedic 2415 McCallie Ave. Chattanooga, TN (423) 624-2696 Shoulder tendinitis is a common overuse injury in sports (such as swimming, baseball and tennis) where the arm is used in an overhead motion. The pain – usually felt at the tip of the shoulder and referred or radiated down the arm – occurs when the arm is lifted overhead or twisted. In extreme cases, pain will be present all of the time and it may even wake you from a deep sleep. The shoulder is a closely fitted joint. The humerus (upper arm bone), the tendons of the rotator cuff that connect to the muscles that lift the arm, and associated bursa (friction reducing membranes), move back and forth through a very tight archway of bone and ligament called the coracoacromial arch. When the arm is raised, the archway becomes smaller and compresses the tendons and bursa. Repetitive use of the arm makes the tendons and bursa prone to injury and inflammation. Bursitis occurs when the bursa becomes inflamed and painful due to compression inside of the coracoacromial arch. Tendinitis occurs when a rotator cuff tendon becomes inflamed, swollen and tender. Symptoms of tendinitis and bursitis usually last for only a few days, but may recur or become chronic. Stages of Tendinitis • Overuse tendinitis. Shoulder motions used during activities like golfing, throwing or overhead lifting may cause repetitive stress within the rotator cuff, leading to irritation, bruising or fraying of the tendon. This can cause shoulder pain and weakness in the joint. -

Rotator Cuff Tear
! Rotator Cuff Tears Description: radiate down the arm towards A rotator cuff tear is a the elbow and up towards the Arthroscopic Repair common cause of shoulder pain neck. Surgery is recommended and disability among adults. Diagnosis: when non-surgical treatments Normally there are 4 rotator cuff Your doctor will perform a have been tried. This tendons that form a covering comprehensive physical technique uses multiple small over the top of the shoulder. examination. Additional tests incisions (portals) and These tendons allow the shoulder may be performed to rule our arthroscopic technology to to move and rotate. Most tears other possible injuries. These visualize and repair the occur in the tests may include x-rays, bone rotator cuff. All-arthroscopic supraspinatus scanning, ultrasound, and repair is usually an outpatient tendon, but magnetic resonance imaging procedure. Suture anchors are other parts of (MRI). placed into the bone and used the rotator to re-attach the tendons to cuff may be Treatment: the bone. The results are involved. Your doctor may recommend comparable if not better than Overuse is the a brief period of anti- to those for mini-open repair most common inflammatory medication and and open repair. cause of tears, however a fall or activity modification. A course of Rehabilitation after surgery is sudden injury can also cause a rehabilitation may also be important. tear. helpful. Injection of a corticosteroid with a local Symptoms: anesthetic may alleviate the Pain often develops slowly symptoms. Anti-inflammatory over a period of time and may be medication should be used worse at night time or with cautiously as these medications overhead activities. -

The Rotator Cuff by Tim Bertelsman & Brandon Steele
The Rotator Cuff by Tim Bertelsman & Brandon Steele. © ChiroUp 2015 Introduction The shoulder is responsible for approximately 16% of all primary care musculoskeletal visits. Rotator cuff injuries are the most common problem to affect the shoulder; accounting for 4.5 million physician office visits per year. The emerging healthcare model dictates that these patients will be directed to the "best" provider- and this guide can help make sure that includes you! Rotator cuff injuries do not occur in isolation, but rather as part of a continuum of dysfunction. The process often begins with a simple muscle imbalance (Upper Crossed Syndrome) that pulls the scapula into a dysfunc- tional movement pattern (Scapular Dyskinesis) and allows for painful pinching of the rotator cuff tendon and associated bursa (Shoulder Anterior Impingement Syndrome- SAIS). Repetitive pinching traumatizes the tendon (Rotator Cuff Tendinopathy), ultimately leading to tear. This continuum is quite predictable. The renowned shoulder orthopedist, Dr Charles Neer, estimated that 95% of rotator cuff tears were from repetitive impingement (SAIS) rather than acute injury. Of those, 100% started with scapular dyskinesis, a component of upper crossed syndrome. Identifying the true “functional” origin of “structural” pathology will dramatically improve your clinical outcomes. This guide helps by summarizing the etiology, assessment and management for each component of the shoulder dysfunction continuum. The appendix provides pictures and descriptions of all relevant orthopedic assessments and exercises. The material is taken from ChiroUp.com- a peer-reviewed, online platform of exceptional evidence-based clinical and business resources that are simple to access and implement today- thereby improving clinical outcomes, patient satisfaction, and practice incomes tomorrow. -

Biomechanical Factors in Rotator Cuff Pathology (2011)
REVIEW ARTICLE Biomechanical Factors in Rotator Cuff Pathology Vasili Karas, BS, Vincent M. Wang, PhD, Aman Dhawan, MD, and Brian J. Cole, MD, MBA muscle is nearly 2.5 times larger than that of the posterior, Abstract: The rotator cuff provides dynamic stability and is critical allowing for more force contraction. Functionally, the to normal shoulder function. Forces generated by the rotator cuff anterior supraspinatus can both internally and externally facilitate the motions involved in activities of daily living and the rotate the humerus, whereas the posterior subregion can more demanding movements of athletics and manual labor. Injury generate external rotation only (Fig. 2).6 In human and pathology of the rotator cuff are common and the unique cadaveric studies, tears have been consistently noted to anatomical and biomechanical characteristics of the cuff contribute 3,7 to the etiology of its injury. This review provides a biomechanical initiate on the anterior aspect of the supraspinatus. In 8 and anatomic context to understanding normal rotator cuff contrast, a recent in vivo ultrasound study by Kim et al function and summarizes recent work describing biomechanical suggests that rotator cuff tears commonly initiate in the implications of cuff pathology. posterior portion of the tendon between the suprapinatus and the infraspinatus. These investigators postulate that the Key Words: rotator cuff, tendon, muscle, collagen, biomechanics, posterior region corresponds to the approximate center of mechanical load, mechanical strain the rotator crescent; an area on the cuff that Burkhart et al9 (Sports Med Arthrosc Rev 2011;19:202–206) suggest bears high load and which degenerates with age. -

(Ii) Rotator Cuff Arthropathy
ARTICLE IN PRESS Current Orthopaedics (2007) 21, 415–421 Available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/cuor MINI-SYMPOSIUM: SHOULDER RECONSTRUCTION (ii) Rotator cuff arthropathy Lennard FunkÃ, John Haines, Ian Trail Wrightington Upper Limb Unit, Wrightington Hospital, Hall Lane, Apley Bridge, Wigan, UK KEYWORDS Summary Rotator cuff Rotator cuff arthropathy is a specific and uncommon disease entity. It affects mainly arthropathy; elderly, but often active people. The exact causes are not known, but there is evidence of Shoulder; genetic predisposition, crystal disease and mechanical factors. There are numerous Deltoid rehabilitation; treatment options for both the pain and dysfunction. Generally, non-operative measures Hyaluronan are successful, if used appropriately and timely. Arthroscopic debridement and washout injections; alone has only short-term benefit, but arthroscopic biceps tenotomy or tenodesis is Arthroscopic shoulder effective in eliminating pain caused by long head of biceps tendon pathology. Anatomical surgery; hemi-arthroplasty, whether stemmed or surface replacement, primarily improves pain. Biceps tenotomy; However, modern designs specifically for cuff tear arthropathy have shown improved Biceps tenodesis; functional results. Surface replacement implants also confer the benefits of being less Surface replacement; invasive and of preserving bone. Reverse total shoulder arthroplasty is a powerful tool for Reverse geometry the elderly patient with unresponsive painful pseudoparalysis. However, it has a high arthroplasty complication rate and should be used with care. & 2007 Elsevier Ltd. All rights reserved. Introduction Charles Neer coined the term ‘‘cuff tear arthropathy’’ in 1977 and published his classic article in 1983.2 Neer Rotator cuff arthropathy is characterised by rotator cuff described the pathoanatomical changes associated with insufficiency, diminished acromiohumeral distance and massive, chronic full-thickness rotator cuff tears, which arthritic changes of the glenohumeral joint.